282790
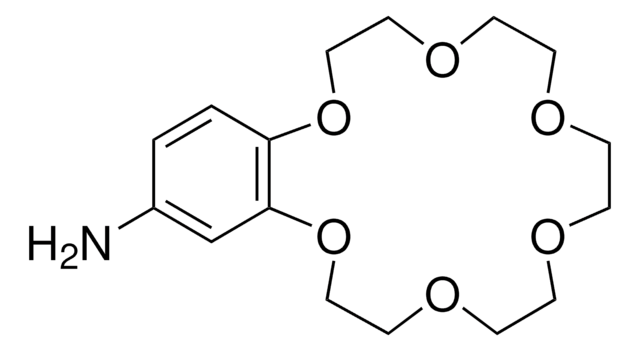
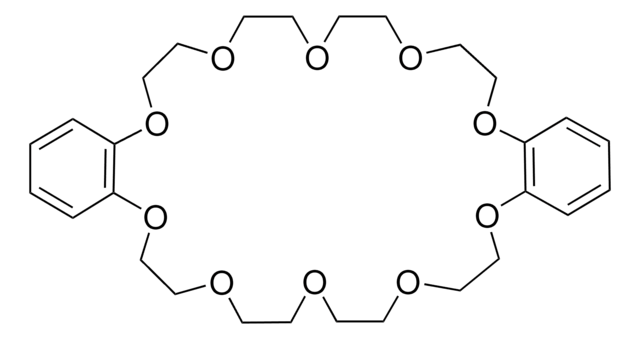
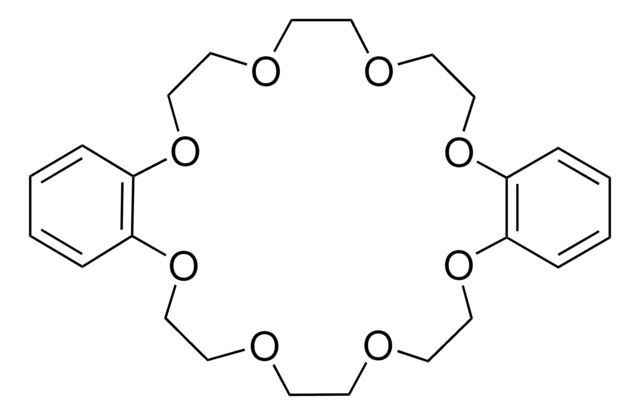
Benzo-15-crown-5
98%
Synonym(s):
2,3,5,6,8,9,11,12-Octahydro-1,4,7,10,13-benzopentaoxacyclopentadecin, 2,3-Benzo-1,4,7,10,13-pentaoxacyclopentadecane, Benzo15C5, Monobenzo-15-crown-5
Sign Into View Organizational & Contract Pricing
Select a Size
Change View
5 X 1 VIAL
CA$1,140.00
About This Item
Empirical Formula (Hill Notation):
C14H20O5
CAS Number:
Molecular Weight:
268.31
Beilstein:
1624106
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
78-80 °C (lit.)
functional group
ether
SMILES string
C1COCCOc2ccccc2OCCOCCO1
InChI
1S/C14H20O5/c1-2-4-14-13(3-1)18-11-9-16-7-5-15-6-8-17-10-12-19-14/h1-4H,5-12H2
InChI key
FNEPSTUXZLEUCK-UHFFFAOYSA-N
General description
Benzo-15-crown-5 is a macrocyclic compound that belongs to the class of crown ethers. It is used as a ligand in metal-catalyzed reactions to enhance the reactivity and selectivity by coordinating with metal cations. For instance, it has been utilized in certain metal-catalyzed cross-coupling reactions, such as Suzuki-Miyaura or Stille reactions, to improve the efficiency and selectivity of the transformations. Benzo-15-crown is also employed as a phase-transfer catalyst in organic synthesis.
Application
Benzo-15-crown-5 can be used as a reactant in the synthesis of a phenol-based benzo-15-crown-5 ether resin. The resulting resin further used for lithium isotope separation, which relies on the selective absorption of the Li-6 isotope.
1 of 4
This Item | 274984 | 188832 | 388424 |
|---|---|---|---|
| assay 98% | assay ≥99.0% | assay 98% | assay 95% |
| Quality Level 100 | Quality Level 200 | Quality Level 200 | Quality Level 100 |
| mp 78-80 °C (lit.) | mp 42-45 °C (lit.) | mp - | mp - |
| functional group ether | functional group ether | functional group ether | functional group ether, hydroxyl |
Still not finding the right product?
Explore all of our products under Benzo-15-crown-5
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yi-Shu Huang et al.
International journal of molecular sciences, 22(4) (2021-03-07)
Epidermal growth factor receptor (EGFR) specific therapeutics is of great importance in cancer treatment. Fcy-hEGF fusion protein, composed of yeast cytosine deaminase (Fcy) and human EGF (hEGF), is capable of binding to EGFR and enzymatically convert 5-fluorocytosine (5-FC) to 1000-fold
Meilyn Rodriguez-Hernandez et al.
Protein and peptide letters, 27(2), 145-157 (2019-10-18)
Glycogen storage disease type III (GSDIII, Cori/Forbes disease) is a metabolic disorder due to the deficiency of the Glycogen Debranching Enzyme (GDE), a large monomeric protein (about 176 kDa) with two distinct enzymatic activities: 4-α-glucantransferase and amylo-α-1,6-glucosidase. Several mutations along
Silvia Carloni et al.
Experimental neurology, 324, 113117-113117 (2019-11-18)
Previous studies have shown that simvastatin (Sim) has neuroprotective effects in a neonatal model of hypoxia-ischemia (HI)-induced brain injury when administered before but not after HI, pointing to the preconditioning (PC)-like effects of the statin. The present study aimed to