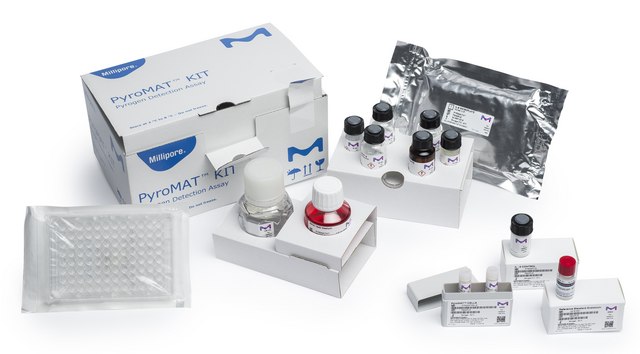
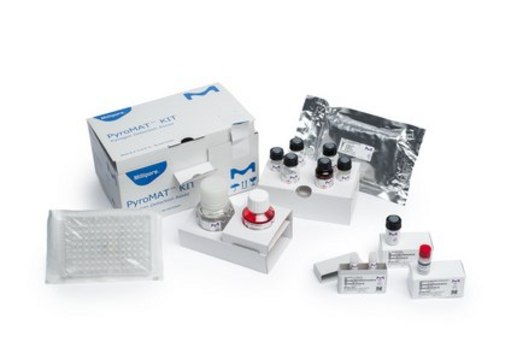
PYR0MATKIT
PyroMAT® kit
Reagents and Interleukin-6 ELISA kit for Monocyte Activation Test (MAT), for Pyrogen testing
About This Item
Recommended Products
Agency
EP Chapter 2.6.30
Quality Level
feature
ready-to-use
packaging
pkg of 1 kit
manufacturer/tradename
PyroMAT®
application(s)
pharmaceutical
pyrogen testing
compatibility
configured for PyroMAT®
shipped in
ambient
storage temp.
2-8°C
suitability
pyrogenic bacteria
Related Categories
1 of 4
This Item | MATHKSA | PYR0MATCELLS | PYR0MATIL6 |
|---|---|---|---|
| manufacturer/tradename PyroMAT® | manufacturer/tradename PyroMAT® | manufacturer/tradename PyroMAT® | manufacturer/tradename PyroMAT® |
| suitability pyrogenic bacteria | suitability pyrogenic bacteria | suitability pyrogenic bacteria | suitability pyrogenic bacteria |
| compatibility configured for PyroMAT® | compatibility configured for PyroDetect System, configured for PyroMAT® System | compatibility configured for PyroMAT® System | compatibility configured for PyroMAT® |
| Quality Level 400 | Quality Level 400 | Quality Level 400 | Quality Level 400 |
| agency EP Chapter 2.6.30 | agency - | agency EP Chapter 2.6.30 | agency EP Chapter 2.6.30 |
| application(s) pharmaceutical | application(s) pharmaceutical | application(s) pharmaceutical | application(s) pharmaceutical |
General description
Application
Features and Benefits
Components
- 96-well plate, Endotoxin free water, RMPI culture medium
- IL-6 ELISA kit and plate
Storage and Stability
Other Notes
Recommended products
Legal Information
comparable product
control
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Eye Irrit. 2 - Met. Corr. 1 - Repr. 1B - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service