BCA1
Bicinchoninic Acid Kit for Protein Determination
for 200-1000 μg/ml protein
Synonym(s):
BCA protein assay
Sign Into View Organizational & Contract Pricing
About This Item
UNSPSC Code:
12352106
NACRES:
NA.32
Pricing and availability is not currently available.
Recommended Products
1 of 4
This Item | 706418 | C112852 | 376906 |
|---|---|---|---|
| assay 97% | assay 97% | assay 98% | assay 98% |
| Quality Level 100 | Quality Level 100 | Quality Level 200 | Quality Level 100 |
| form powder | form powder | form liquid | form solid |
| mp 146-151 °C | mp 170-175 °C | mp 19 °C (lit.) | mp 122-124 °C (lit.) |
| functional group carboxylic acid | functional group carboxylic acid, hydroxyl, phenyl | functional group - | functional group carboxylic acid, hydroxyl, phenyl |
Application
Features and Benefits
- Simple, sensitive colorimetric assay for proteins
- Two stable reagents are used for the working solution
- Faster and easier than the Lowry protein assay
- Compatible with many detergents both ionic and nonionic
- Less variation between different proteins than the Bradford dye-binding assay
- Adaptable for use in microwell plates
Principle
Proteins reduce alkaline Cu(II) to Cu(I) in a concentration-dependent manner. Bicinchoninic acid is a highly specific chromogenic reagent for Cu(I), forming a purple complex with an absorbance maximum at 562 nm. The absorbance is directly proportional to protein concentration. This is an alternative to the Folin-Ciocalteu reagent for protein determination.
Kit Components Only
Product No.
Description
- Bicinchoninic Acid Solution 1 L
- 4%(w/v) CuSO4 · 5H2O Solution 25 mL
- Protein Standard Solution 5 x 1
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
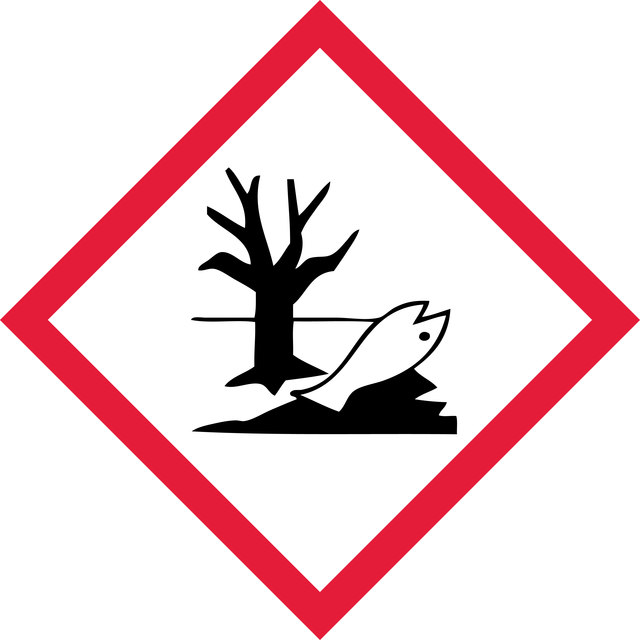
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Met. Corr. 1
Storage Class Code
8A - Combustible corrosive hazardous materials
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Christiane Kiske et al.
Journal of agricultural and food chemistry, 67(4), 1187-1196 (2019-01-04)
The enantiomers of a homologous series (C6-C10) of 2-mercapto-4-alkanones were obtained by lipase-catalyzed kinetic resolution of the corresponding racemic 2-acetylthio-4-alkanones. Their configurations were assigned via vibrational circular dichroism and 1H NMR anisotropy based methods. Odor thresholds and odor qualities were
Chunze Li et al.
Chemical research in toxicology, 15(10), 1309-1317 (2002-10-22)
Chemically reactive species formed from the metabolism of carboxylic acid-containing compounds have been proposed as mediators of their toxic side-effects. Two alternative metabolic pathways known to be involved in the generation of reactive acylating metabolites of carboxylic acids are acyl
D Ahmad et al.
Chirality, 6(5), 365-371 (1994-01-01)
The significance of disturbances of lipid metabolism caused by xenobiotic acyl-CoAs as possible causes of peroxisomal proliferation has been studied with the enantiomers of 2-phenylpropionic acid (2-PPA), the (R)-enantiomer of which is converted to the acyl-CoA in rats while its
A J Hutt et al.
Chirality, 5(8), 596-601 (1993-01-01)
The metabolism of (R,S)-ibuprofen has been investigated in 24 microbial cultures. Of these Cunninghamella elegans, Mucor hiemalis, and Verticillium lecanii catalyzed the oxidation of the drug to 2-[4-(2-hydroxy-2-methylpropyl)phenyl]propionic acid, a known mammalian metabolite. The extent of metabolism was greatest with
D K Bhattacharyya et al.
The Journal of biological chemistry, 271(4), 2179-2184 (1996-01-26)
Examination of the crystal structure of the ovine prostaglandin endoperoxide synthase-1 (PGHS-1)/S- flurbiprofen complex (Picot, D., Loll, P.J., and Garavito, R.M. (1994) Nature 367, 243-2491) suggests (a) that the carboxyl group of arachidonic acid interacts with the arginino group of
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service