P0052
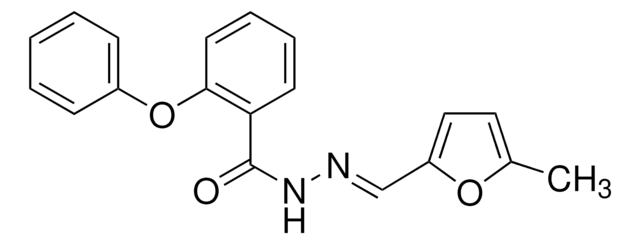
PNU-74654
≥98% (HPLC), solid
Synonym(s):
Benzoic acid, 2-phenoxy-, 2-[(5-methyl-2-furanyl)methylene]hydrazide
About This Item
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
solid
color
white to off-white
solubility
DMSO: >10 mg/mL
storage temp.
2-8°C
SMILES string
Cc1ccc(\C=N\NC(=O)c2ccccc2Oc3ccccc3)o1
InChI
1S/C19H16N2O3/c1-14-11-12-16(23-14)13-20-21-19(22)17-9-5-6-10-18(17)24-15-7-3-2-4-8-15/h2-13H,1H3,(H,21,22)/b20-13+
InChI key
JJEDWBQZCRESJL-DEDYPNTBSA-N
1 of 4
This Item | SML1970 | SML1965 | SML0002 |
|---|---|---|---|
| assay ≥98% (HPLC) | assay ≥98% (HPLC) | assay ≥98% (HPLC) | assay ≥98% (HPLC) |
| form solid | form powder | form powder | form powder |
| Quality Level 100 | Quality Level 100 | Quality Level - | Quality Level 100 |
| storage temp. 2-8°C | storage temp. 2-8°C | storage temp. 2-8°C | storage temp. 2-8°C |
| solubility DMSO: >10 mg/mL | solubility DMSO: 2 mg/mL, clear | solubility DMSO: 15 mg/mL, clear | solubility DMSO: >25 mg/mL |
| color white to off-white | color light yellow to dark orange | color white to brown | color red to brown |
Application
- as a β-catenin signaling inhibitor to study its effects on the funnel cartilage differentiation in Sepia sp. embryos[1]
- as a Wnt signaling inhibitor to study its effects on glycogen synthase kinase 3β (GSK-3β) inhibition in prostate cancer cell lines[2]
- as a Wnt signaling inhibitor to study its effects on leptin-induced cell proliferation in breast cancer cells[3]
Biochem/physiol Actions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Information on fatty acid synthesis and metabolism in cancer cells. Learn how proliferatively active cells require fatty acids for functions such as membrane generation, protein modification, and bioenergetic requirements. These fatty acids are derived either from dietary sources or are synthesized by the cell.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service