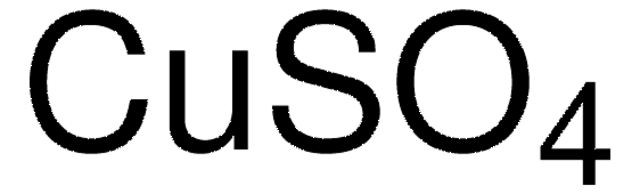1.02784
Copper sulfate solution
c(CuSO₄)=0.1 mol/l, ready-to-use volumetric solution for titration, Titriplex®
Synonyme(s) :
Copper(II) Sulfate Aqueous Solution
About This Item
Produits recommandés
Product Name
Copper sulfate solution, c(CuSO4)=0.1 mol/l, Titripur®
Niveau de qualité
Gamme de produits
Titripur®
Forme
liquid
Qualité
Analyzed in our ISO 17025 accredited QC lab
Capacité de réaction
reaction type: Redox Reactions
Concentration
0.1 M
Technique(s)
titration: suitable
pH
4.2 (20 °C in H2O)
Densité
1.02 g/m3 at 20 °C
1 of 4
Cet article | C2284 | 908940 | 61230 |
|---|---|---|---|
| reaction suitability reaction type: Redox Reactions | reaction suitability - | reaction suitability - | reaction suitability - |
| technique(s) titration: suitable | technique(s) - | technique(s) - | technique(s) - |
| product line Titripur® | product line - | product line ReagentPlus®, Redi-Dri™ | product line - |
| form liquid | form liquid | form powder | form powder |
| quality Analyzed in our ISO 17025 accredited QC lab | quality - | quality free-flowing | quality - |
| concentration 0.1 M | concentration 4 % (w/v) (prepared from copper (II) sulfate pentahydrate) | concentration - | concentration - |
Application
- Self-organized tubular structures as platforms for quantum dots.: This study explores the formation of self-organized tubular structures that can potentially serve as platforms for quantum dots. These structures are formed using copper sulfate solutions in an electrochemical setting, highlighting the versatility and utility of copper sulfate in analytical chemistry applications such as quantum dot technology (Makki R et al., 2014).
- Extended space charge in concentration polarization.: The research discusses the phenomenon of extended space charge within the context of concentration polarization, a critical aspect in electrochemistry and colloid science. Copper sulfate solutions are utilized to study the electrochemical properties and dynamics under various conditions, providing insights into material behavior at the microscale (Rubinstein I et al., 2010).
Caractéristiques et avantages
This volumetric solution is analyzed by our calibration laboratory D-K-15185-01-00 which is accredited according to DIN EN ISO/IEC 17025 for analysis of amount-of-substance concentrations in volumetric solutions by DAkkS (Deutsche Akkreditierungsstelle - German National Accreditation Body). The accreditation certificate can be found at www.sigmaaldrich.com/ISO17025.
Conditionnement
Remarque sur l'analyse
Amount-of-substance concentration 0.0995 - 0.1005 mol/L
Measurement uncertainty ± 0.0003 mol/L
Traceability NIST SRM
The concentration is determined by volumetric titration and refers to 20°C.
The amount-of-substance concentration of this volumetric solution is determined with standardized titriplex-III solution (article number 1.08431). The titriplex-III solution is standardized and traceable to a primary standard reference material (SRM) from the National Institute of Standards and Technology, Gaithersburg, USA (NIST SRM 682 Zinc) by means of volumetric standard Zinc (article number 1.02409), certified reference material according to ISO 17034, analyzed by our accredited calibration laboratory of Merck KGaA, Darmstadt, Germany according to DIN EN ISO/IEC 17025. The uncertainty is expressed as expanded measurement uncertainty with a coverage factor k=2 covering a confidence level of 95%.
Note: The titer is a correction factor to correct for variations of the volumetric solution, the titration equipment, the temperature and other laboratory conditions. For correct titration results it is recommended to determine a titer with the laboratory specific equipment and under laboratory specific conditions directly after opening a new bottle and at regular time intervals.
Informations légales
Mentions de danger
Conseils de prudence
Classification des risques
Aquatic Chronic 3
Code de la classe de stockage
12 - Non Combustible Liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Besoin d'un modèle de COA ?
Ceci est un modèle de certificat d'analyse (COA pour Certificate of Analysis) et peut ne pas être représentatif d'un lot récemment fabriqué de ce produit spécifique.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique