P4850
Proteinase K from Tritirachium album
buffered aqueous glycerol solution, ≥800 units/mL
Synonym(s):
Endopeptidase K
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
grade
Molecular Biology
Quality Level
form
buffered aqueous glycerol solution
mol wt
28.93 kDa
concentration
≥10 mg/mL
≥800 units/mL
impurities
≤0.5 ppm DNA (PicoGreen® assay)
foreign activity
DNase, Nickase and RNase, none detected
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
Application
Proteinase K from Tritirachium album has been used:
- in bovine endometrial epithelial cells for herpes viral DNA extraction
- for viral RNA extraction from nasal swabs
- as a component of phase lock and direct PCR lysis buffer
The product has been used to study its pre-treatment effects on the silk fibroin. The aspects analysed in this study included the crystallographic properties of hydroxyapatite (HAp), and the microstructure and microhardness of the composites. The enzyme has also been used to facilitate the access of probes to rRNA using FISH techniques to detect pathogenic Staphylococcus aureus.
Useful for the proteolytic inactivation of nucleases during the isolation of DNA and RNA.
Useful for the proteolytic inactivation of nucleases during the isolation of DNA and RNA.
Removes endotoxins that bind to cationic proteins such as lysozyme and ribonuclease A.
Reported useful for the isolation of hepatic, yeast, and mung bean mitochondria
Determination of enzyme localization on membranes
Treatment of paraffin embedded tissue sections to expose antigen binding sites for antibody labeling.
Digestion of proteins from brain tissue samples for prions in Transmissible Spongiform Encephalopathies (TSE) research.
Removes endotoxins that bind to cationic proteins such as lysozyme and ribonuclease A.
Reported useful for the isolation of hepatic, yeast, and mung bean mitochondria
Determination of enzyme localization on membranes
Treatment of paraffin embedded tissue sections to expose antigen binding sites for antibody labeling.
Digestion of proteins from brain tissue samples for prions in Transmissible Spongiform Encephalopathies (TSE) research.
Biochem/physiol Actions
Proteinase K has a broad specificity and degrades many proteins even in the native state. It mainly cleaves the peptide bond adjacent to the carboxyl group of aliphatic and aromatic amino acids with blocked α-amino groups. The molecular weight of proteinase K from amino acid sequence is found to be 28,930 Da and from SDS-PAGE, it is found to be 28,500 Da. The optimum pH is between 7.5-9.0 and its isoelectric point is 8.9. Ca2+ (1-5 mM) is required for its activation. Proteinase K is inhibited by DIFP (diisopropylfluorophosphate) or PMSF (phenylmethylsulfonyl fluoride).
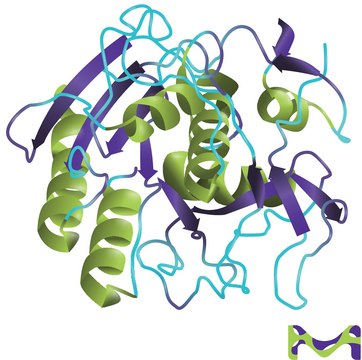
Proteinase K is a stable and highly reactive serine protease. Evidence from crystal and molecular structure studies indicates the enzyme belongs to the subtilisin family with an active-site catalytic triad (Asp39-His69-Ser224). It is stable in a broad range of environments: pH, buffer salts, detergents (SDS), and temperature. In the presence of 0.1-0.5% SDS, proteinase K retains activity and will digest a variety of proteins and nucleases in DNA preparations without compromising the integrity of the isolated DNA.
Unit Definition
One unit will hydrolyze urea-denatured hemoglobin to produce color equivalent to 1.0 μmole of tyrosine per min at pH 7.5 at 37 °C (color by Folin-Ciocalteu reagent).
Physical form
Solution in 40% glycerol (v/v) containing 10 mM Tris-HCl, pH 7.5, with 1 mM calcium acetate.
Legal Information
PicoGreen is a registered trademark of Life Technologies
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service