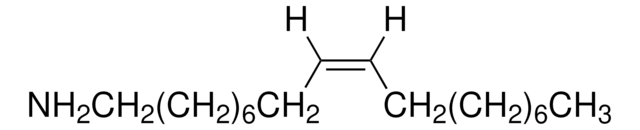
385395
Acetaldehyde dimethyl acetal
95%
Synonym(s):
1,1-Dimethoxyethane, Dimethyl acetal
Sign Into View Organizational & Contract Pricing
Select a Size
1 EACH
CA$138.00
Select a Size
Change View
1 EACH
CA$138.00
About This Item
Linear Formula:
CH3CH(OCH3)2
CAS Number:
Molecular Weight:
90.12
Beilstein:
1697039
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39021102
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
3.1 (vs air)
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.367 (lit.)
bp
64 °C (lit.)
density
0.852 g/mL at 25 °C (lit.)
functional group
acetal
ether
SMILES string
COC(C)OC
InChI
1S/C4H10O2/c1-4(5-2)6-3/h4H,1-3H3
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Kinetics of oxidative degradation of acetaldehyde dimethyl acetal has been studied by pulse radiolysis.[1] It also undergoes addition with various ketones, esters, amides and thioesters in the presence of silyl trifluoromethanesulfonates and an amine base.[2] Lewis acid-mediated reaction of the titanium enolate of (S)-N-acetyl-4-isopropyl-1,3-thiazolidine-2-thione with acetaldehyde dimethyl acetal has been studied.[3]
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
1.4 °F - closed cup
Flash Point(C)
-17 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Karl J Bonney et al.
The Journal of organic chemistry, 76(1), 97-104 (2010-12-01)
9-Oxabicyclo[6.1.0]non-4-ene (1) undergoes intramolecular bromonium ion-assisted epoxide ring-opening using N-bromosuccinimide via a presumed oxonium ion that is subject to stereospecific, nonregioselective capture with added external nucleophiles producing novel bicyclo[4.2.1] and bicyclo[3.3.1] ethers. Carboxylic acids (as catalyzed by tetramethylguanidine), alcohols, water
Stereoselective synthesis of cyclic ethers via bromine assistedepoxide ring expansion.
Davies SG, et al.
Tetrahedron Letters, 26(11), 1461-1464 (1985)
Transannular oxygen participation in halofluorination reactions of 9-oxabicyclo [6.1. 0] non-4-ene [1].
Haufe G, et al.
Journal of Fluorine Chemistry, 46(1), 83-95 (1990)
High-yielding, two-step 18F labeling strategy for 18F-PARP1 inhibitors.
Edmund J Keliher et al.
ChemMedChem, 6(3), 424-427 (2011-03-02)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service