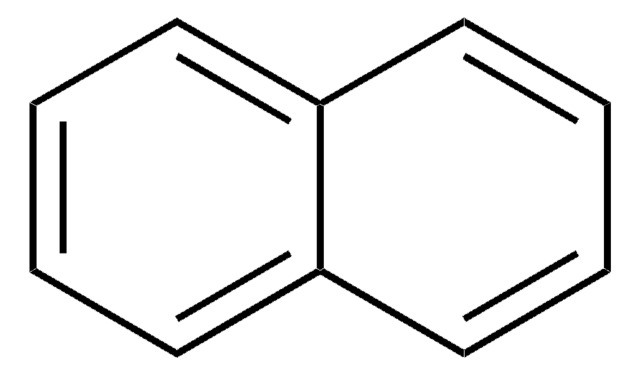
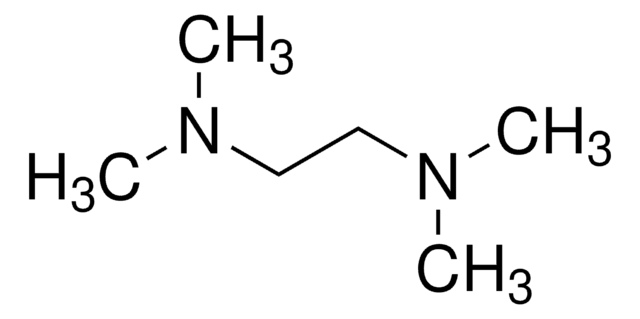
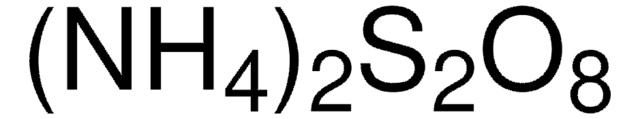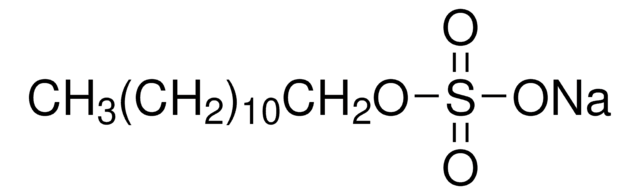G5516
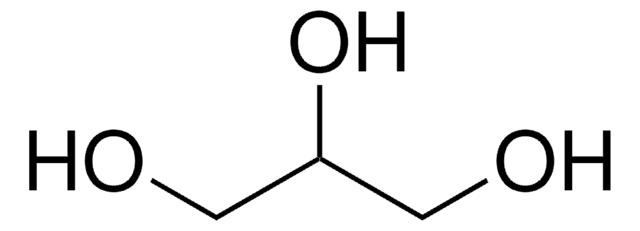
Glycerol
Molecular Biology, ≥99.0%
Synonym(s):
1,2,3-Propanetriol, Glycerin
About This Item
reagent grade
cryopreservation: suitable
microbiology
sample preservation
Recommended Products
grade
Molecular Biology
reagent grade
Quality Level
vapor density
3.1 (vs air)
vapor pressure
<1 mmHg ( 20 °C)
Assay
≥99.0%
form
viscous liquid
autoignition temp.
698 °F
expl. lim.
≥2.7-19.0 %
technique(s)
MALDI-MS: suitable
cryopreservation: suitable
impurities
<5 ppm Iron
<5 ppm heavy metal
<5 ppm magnesium
Looking for similar products? Visit Product Comparison Guide
Related Categories
1 of 4
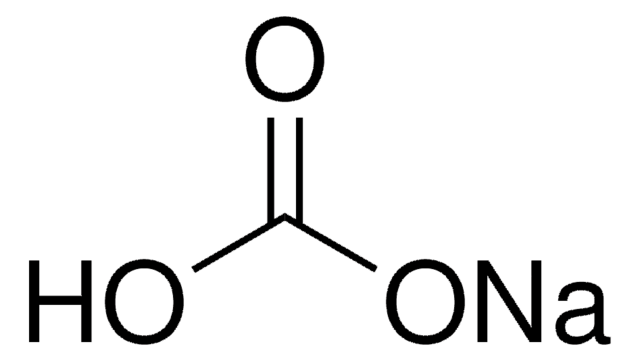
This Item | 49767 | 1.37028 | G2025 |
|---|---|---|---|
| application(s) life science and biopharma | application(s) - | application(s) liquid formulation | application(s) - |
| grade for molecular biology, reagent | grade for molecular biology | grade ACS reagent | grade - |
| assay ≥99.0% | assay ≥99.5% (GC) | assay ≤98.0-101.0% (calculated on anhydrous substance, alkalimetric), ≥99.5% (calculated on anhydrous substance, GC) | assay ≥99% (GC) |
| technique(s) MALDI-MS: suitable, cryopreservation: suitable | technique(s) - | technique(s) electrophoresis: suitable | technique(s) cell culture | insect: suitable, cell culture | mammalian: suitable |
| Quality Level 200 | Quality Level 200 | Quality Level 500 | Quality Level 300 |
| solubility water: 1 mL/mL, clear, colorless | solubility H2O: 5 M at 20 °C, clear, colorless | solubility - | solubility - |
General description
Application
- a component of mounting medium for immunofluorescence
- a supplement during cell culture of Mycobacterium tuberculosis and Mycobacterium avium.[2]
- a fuel during the designing of enzymatic biofuel cells.[3]
- a liquid composite matrix with 4-HCCA and 3-aminoquinoline for analysis of neutral and acidic glycans.[4][5]
- a matrix for fast atom bombardment MS.[4][5]
Biochem/physiol Actions
Features and Benefits
Other Notes
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
390.2 °F - Pensky-Martens closed cup
Flash Point(C)
199 °C - Pensky-Martens closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service