Quality is Built into Every MILLIPLEX® Multiplex Kit
For more than 20 years, we have offered the benefits of MILLIPLEX® multiplex immunoassays, containing all the components you need to detect multiple analytes simultaneously. We provide the largest selection of multiplex immunoassays, analytes, and species to meet the needs of investigators across multiple research areas. Rely on the quality we build into each MILLIPLEX® multiplex kit to produce results you trust.

Expert Research and Development
With over four decades of immunoassay development and manufacturing expertise, MILLIPLEX® kits are developed and quality tested for accurate, reproducible, and biologically relevant analyte measurements.

Dedicated Support
Committed care when and where you need it, with knowledgeable in-house and field-based scientific support for assays and instruments.

Skilled Manufacturing and Quality Control
MILLIPLEX® multiplex kits are manufactured in facilities that are ISO 9001:2015 compliant.

Optimized Kit Components and Reagents
MILLIPLEX® kit components are produced with quality raw materials and are pre-optimized for easy use and minimal preparation steps.
Lot-to-Lot Reproducibility
Reproducibility across multiple component lots is important to ensure reliable results over time.
See how one of our panels demonstrated superior lot-to-lot consistency of cytokine measurement.
Standard Curves are Checked for Each Analyte Across Kit Lots
Each new lot of MILLIPLEX® standards and quality controls (QCs) are compared to the previous lot and a reference lot, our internal gold standard, to ensure lot-to-lot reproducibility and consistency (Figure 1).

Figure 1. Gold Standard lot compared to three subsequent lots (1K-3K) of the analyte Human IL-6 for Cat. No. HSTCMAG-28SK.
Additionally, each lot is tested after lyophilization to confirm that there has been no loss due to the lyophilization process. Full standard curve characteristics and relative potency of analytes are maintained within the specifications of the reference lot (Figure 2).
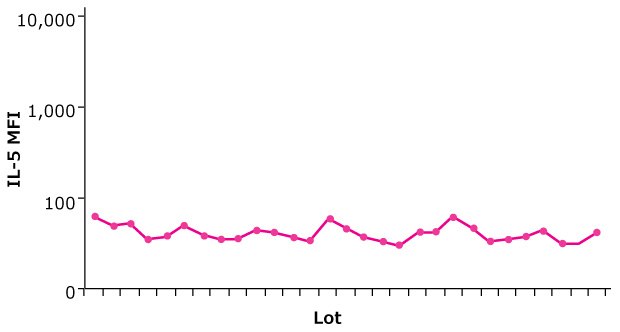
Figure 2. Relative potency of an analyte standard is maintained from lot to lot within specifications. Trend chart shows consistent MFI values for a single IL-5 standard curve point across 29 lots of a MILLIPLEX® panel (Cat. No. HCYTOMAG-60K); ± 10% of reference lot.
Since MILLIPLEX® panels stand the test of time, new standard lots are periodically assigned to be the fresh reference lot against which subsequent lots are compared. All data is compiled in a single database and trend charts are maintained. Other suppliers compare new standard lots to previous lots, without a reference lot, which makes it difficult to compare data from multiple lots since standard curve point values may vary with each new lot and assay drift may occur.
The Importance of Standard Curve Potency
Analyte standard curves from all lots should overlap as closely as possible. Maintaining standard curve potency acceptance criteria is important because it helps to avoid common problems which negatively impact the calculation of analyte concentration in samples (Figure 3). Problematic standard and component lots are never released for sale.

Figure 3. Example of problems that may arise when producing new standard lots, highlighting the importance of standard curve potency.
Quality Controls to Verify Assay Performance
We include two QCs and QC range sheets to qualify and verify assay performance. Assay results for these QCs are to be checked against the provided QC range sheet values to ensure proper pipetting and assay setup have been accomplished. QC values are based on a minimum of six assays run by at least three different operators. The use of high and low QC values serves as an additional checkpoint in case there was user error associated with hydrating or diluting standards.
Additionally, QCs are important for:
- Translational studies that require more validation, ensuring that the data are reproducible across kit lots
- Comparing data across multiple sites
- Comparing assay results from multiple technicians
As with other MILLIPLEX® components, trend charts are maintained for QC ranges across multiple lots. As an added measure, it is recommended that customers include experiment-relevant sample QCs in each assay (Figure 4).

Figure 4. 96-well plate layout shows the placement of standards, QCs, and the recommended experiment-relevant sample QCs.
Other MILLIPLEX® Components
Other components that help enhance the quality of MILLIPLEX® kits include:
Serum Matrix
Because blood is a complex matrix that contains proteins that may interfere with the accurate measurement of the desired analyte, using an optimized serum matrix in the standard curve when measuring analytes secreted in serum or plasma:
- Significantly improves the accuracy of measurement
- More accurately simulates the conditions of the native analyte present in serum or plasma
- Mimics the environment of native analytes in serum or plasma
Bead Diluent
A percentage of the healthy population samples contain heterophilic antibodies that can non-specifically bind to the capture and detection antibodies simultaneously, generating a false positive signal. MILLIPLEX® bead diluents contain a cocktail of proprietary reagents that significantly reduce this false signal without reducing the analyte measurement.
Detection Antibody Cocktail
MILLIPLEX® detection antibody cocktails are designed to yield consistent analyte profiles within the panel, lot-to-lot, regardless of plex size (Figure 5).

Figure 5. Consistent analyte profiles are seen when comparing multiplex and singleplex assays from the same MILLIPLEX® panel, human G-CSF shown.
MILLIPLEX® Assay Development
From academia to contract research to big pharma, we meet the ever-increasing demand for high-quality assays to achieve reproducible results.
We consider all the factors that go into assay development, including:
- Detection and Sensitivity
- Performance in a Sample Matrix
- Specificity
- Selectivity
- Precision and Accuracy
- Linearity
- Stability
- Cross-Talk
- Lot-to-Lot Reproducibility
- Vendor Support
We set and define our MILLIPLEX® assay specifications during kit development. Verification data is provided in each kit protocol. Every MILLIPLEX® kit includes all the optimized reagents and instructions needed to run the assay and read the assay plate on your Luminex® instrument.
In addition to assay specifications listed in the MILLIPLEX® protocols, we evaluate additional performance criteria during our kit development and assay verification process, including dilutional linearity, kit stability, and sample behavior (e.g., sample detectability and stability) described in Table 1.
| Assay Specification | Description |
|---|---|
| Antibody selectivity, specificity, and cross-reactivity | Test each antibody for detection of other analytes within the same panel*; test species cross-reactivity** |
| Gold standard | Reference calibrator specifications defined |
| Standard curve range* | Concentration for each standard dilution |
| Sensitivity* | Minimum detectable concentration (MinDC+2SD) |
| Precision of control standards* | Intra-assay within 15% CV (SD/Mean) Inter-assay within 20% CV (SD/Mean) |
| Accuracy* | The recovery of spiked standards ranging from low, medium, and high concentrations in samples expressed as a percentage |
| Dilution linearity** | The back-calculated value of each point in the dilution series expressed as a percentage of the starting concentration |
| Verified sample types* | Typically serum, plasma, tissue/cell culture; for specific kits, CSF (cerebrospinal fluid), or urine |
| Sample testing** | For biological relevance, detection of analyte within range of assay |
| Sample dilution* | Optimized for all analytes within each kit |
| Serum matrix* | An optimized proprietary serum matrix is developed for each kit where a matrix effect has been observed upon spike/recovery analysis; added to standards when assaying serum/plasma samples in order to simulate sample environment |
| Consistent performance** | Whether assayed in multiplex or singleplex |
| Stability testing* | For shipping and storage of kit components; premixed beads; sample/analyte freeze/thaw cycles |
| Quality controls and QC ranges*** | Recombinant protein at two dilutions are run in repeat assays to establish a target QC range; used to verify assay performance compared to kit specifications; user should include experiment-specific QC sample controls in assay |
| * Kit Protocol ** Contact Technical Support *** Kit Insert | |
To continue reading please sign in or create an account.
Don't Have An Account?