SML0337
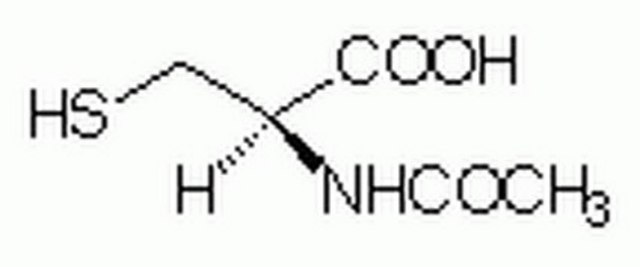
S-Allyl-L-cysteine
≥98% (HPLC)
동의어(들):
L-Deoxyalliin, S-allyl-cysteine, S-allylcysteine, SAC
로그인조직 및 계약 가격 보기
About This Item
실험식(Hill 표기법):
C6H11NO2S
CAS Number:
Molecular Weight:
161.22
MDL number:
UNSPSC 코드:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.77
가격 및 재고 정보를 현재 이용할 수 없음
추천 제품
1 of 4
이 품목 | 1013057 | 106425 | SML3436 |
|---|---|---|---|
| form powder | form - | form solid | form powder |
| assay ≥98% (HPLC) | assay - | assay ≥98% (titration) | assay ≥98% (HPLC) |
| Quality Level 100 | Quality Level - | Quality Level 200 | Quality Level 100 |
| storage temp. −20°C | storage temp. −20°C | storage temp. 15-25°C | storage temp. -10 to -25°C |
| solubility H2O: >10 mg/mL | solubility - | solubility water: 50 mg/mL | solubility 2 mg/mL, clear (0.1N HCl |
| color white to beige | color - | color white | color white to beige |
일반 설명
S-Allyl-L-cysteine has anti-inflammatory properties. It inhibits prooxidant enzymes and chelates metals.[1] S-Allyl-L-cysteine exhibits hypoglycemic[2] and cholesterol-lowering effects.[3] It prevents apoptosis, lowers the activity of inducible nitric oxide synthase and blocks nuclear factor kappa B activity.[3]
생화학적/생리학적 작용
S-allyl-L-cysteine is a sulfur containing amino acid found in garlic with antioxidant, anti-cancer, antihepatotoxic, neuroprotective and neurotrophic activity.
S-allyl-L-cysteine is a sulfur containing amino acid found in garlic with antioxidant, anti-cancer, antihepatotoxic, neuroprotective and neurotrophic activity. S-allyl-L-cysteine has potent activity in several animal models of ischemic injury and Alzhemer′s disease.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Alon Angert et al.
Scientific reports, 9(1), 741-741 (2019-01-27)
Carbonyl sulfide (COS) is the major long-lived sulfur bearing gas in the atmosphere, and is used to estimate the rates of regional and global (both past and current) photosynthesis. Sulfur isotope measurements (34S/32S ratio, δ34S) of COS may offer a
Solution-processed and high-performance organic solar cells using small molecules with a benzodithiophene unit.
Zhou J, et al.
Journal of the American Chemical Society, 135(23), 8484-8487 (2013)
Sukyoung Hwang et al.
Scientific reports, 5, 11201-11201 (2015-06-19)
The growth kinetics of polymer thin films prepared by plasma-based deposition method were explored using atomic force microscopy. The growth behavior of the first layer of the polythiophene somewhat differs from that of the other layers because the first layer
Thiophene Polymer Semiconductors for Organic Thin-Film Transistors
Ong BS, et al.
Chemistry?A European Journal, 14(16), 4766-4778 (2008)
Thiophene, Benzo [b] thiophene and Dibenzo [b, d] thiophene as Precursors to Highly Conjugated Organosulfur Compounds.
Bianchini, Claudio and Meli, Andrea
Synlett, 1997(06), 643-649 (1997)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.