SML3737
Octyl-(S)-2HG
≥98% (HPLC)
동의어(들):
(2S)-2-Hydroxyglutarate octyl ester, (2S)-Octyl-α-hydroxyglutarate, (S)-4-Hydroxy-5-(octyloxy)-5-oxopentanoic acid, 1-Octyl-L-2-hydroxyglutarate, 2S-Hydroxy-pentanedioic acid, 1-octyl ester, L-Octyl-2HG, L2HG, Octyl-(S)-2-hydroxyglutarate, Octyl-L-2HG, S-2HG octyl ester
About This Item
추천 제품
Quality Level
분석
≥98% (HPLC)
양식
powder
색상
white to beige
solubility
DMSO: 2 mg/mL, clear
저장 온도
-10 to -25°C
SMILES string
O[C@@H](CCC(=O)O)C(=O)OCCCCCCCC
InChI
1S/C13H24O5/c1-2-3-4-5-6-7-10-18-13(17)11(14)8-9-12(15)16/h11,14H,2-10H2,1H3,(H,15,16)/t11-/m0/s1
InChI key
UJZOKTKSGUOCCM-NSHDSACASA-N
관련 카테고리
1 of 4
이 품목 | C8696 | C0715 | SRP0289 |
|---|---|---|---|
| Gene Information human ... CTSD(1509) | Gene Information human ... CTSD(1509) | Gene Information human ... CTSD(1509) | Gene Information human ... CTSB(1508) |
| technique(s) activity assay: suitable | technique(s) - | technique(s) immunohistochemistry (formalin-fixed, paraffin-embedded sections): 1:200 using human breast carcinoma tissue, indirect ELISA: suitable, microarray: suitable, western blot: 1:1,000 using human breast carcinoma cell line extract | technique(s) activity assay: suitable |
| assay ≥95% (SDS-PAGE) | assay - | assay - | assay ≥90% (SDS-PAGE) |
| biological source human | biological source - | biological source mouse | biological source human |
| application(s) life science and biopharma | application(s) - | application(s) - | application(s) life science and biopharma |
| form lyophilized | form lyophilized powder | form - | form aqueous solution |
생화학적/생리학적 작용
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.![[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride](/deepweb/assets/sigmaaldrich/product/structures/471/091/6faa29b1-bf8a-4d87-90b2-4cc55e082620/640/6faa29b1-bf8a-4d87-90b2-4cc55e082620.png)


![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)

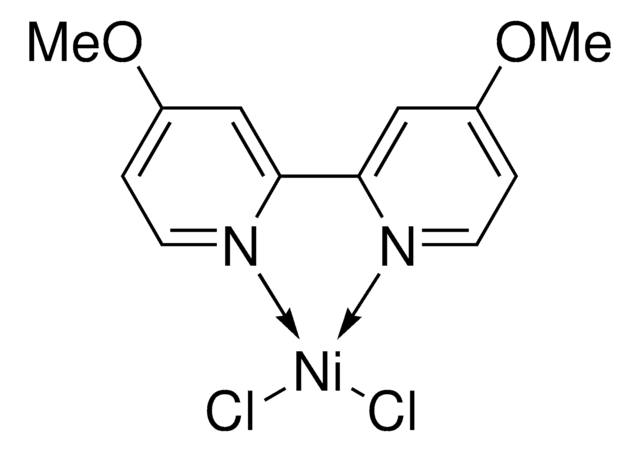
![[4,4′-Dimethyl-2,2′-bipyridine]nickel(II) dichloride hydrate ≥95%](/deepweb/assets/sigmaaldrich/product/structures/714/807/6f838a65-cb7b-400f-bdb3-3e207bb2ddc4/640/6f838a65-cb7b-400f-bdb3-3e207bb2ddc4.png)

![[Ni(dtbbpy)(H2O)4]Cl2](/deepweb/assets/sigmaaldrich/product/structures/777/629/15c13300-e874-4abd-8bd4-8b2bb4864570/640/15c13300-e874-4abd-8bd4-8b2bb4864570.png)




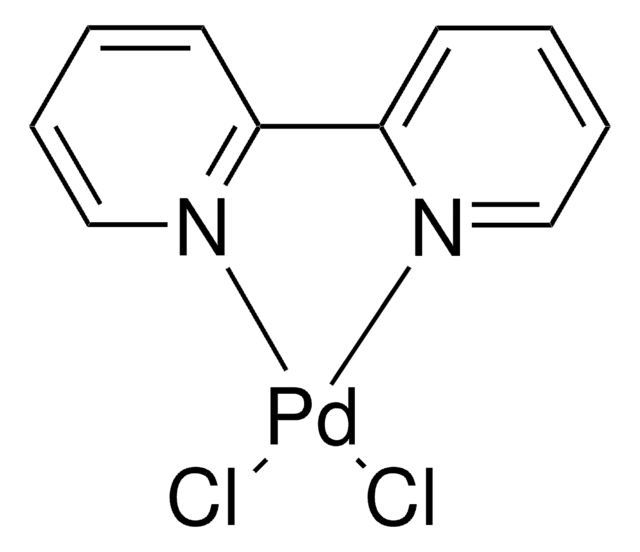
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloronickel(II) 97%](/deepweb/assets/sigmaaldrich/product/structures/274/566/a60d6584-163a-4c41-a738-60f8e4d524fa/640/a60d6584-163a-4c41-a738-60f8e4d524fa.png)
![[1,3-Bis(diphenylphosphino)propane]dichloronickel(II)](/deepweb/assets/sigmaaldrich/product/structures/844/065/af07f787-c6a3-4a6e-a22b-47a933c73978/640/af07f787-c6a3-4a6e-a22b-47a933c73978.png)
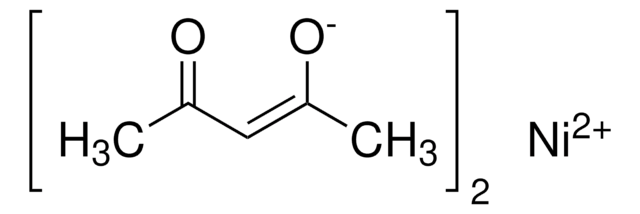
![[1,2-Bis(diphenylphosphino)ethane]dichloronickel(II)](/deepweb/assets/sigmaaldrich/product/structures/707/956/483e7a6e-5fb5-4e39-abd1-ecf33ccab3cf/640/483e7a6e-5fb5-4e39-abd1-ecf33ccab3cf.png)
