Molecular Self-Assembly on Gold Using Thioacetates
Kanth Josyula, Mitesh Patel, Kaushik Patel, Scott Batcheller
Materials Science 6000 N Teutonia Milwaukee, WI 53209
SELF ASSEMBLY - APPLICATIONS
Over the last twenty years, Self Assembled Monolayers (SAMs) have been widely used to control surface properties in various research fields primarily using thiols and disulfides.
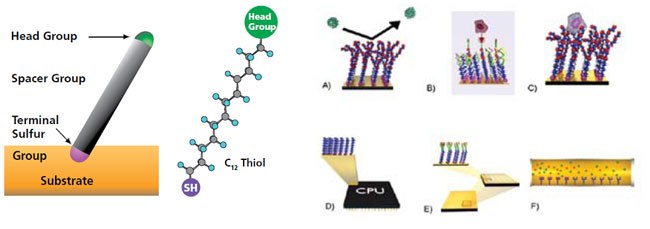
The sulfur atom from the thiol group is able to assemble molecules to surfaces, such as gold. A pre-determined head group can also be displayed.
Example applications include: a) non-foluing surfaces b) SAMs with specific binding receptors c) cell supports for native cell growth d) molecular electronics e) microarrays and f) separations.
Materials for SAMs - Challenges
- High purity thiols are essential for consistent, ordered layers with well-defined thickness. Impure thiols result in thinner and more disordered monolayers.
- Higher complexity thiols, with several functional groups, are becoming popular as a way to introduce complex functionality to SAMs. The stability of these complex thiols becomes a major challenge, as they are prone to forming oxidation impurities. This may compromise storage stability and performance reliability in research applications.
- SAMs may alternatively be built from disulfides; however, this often results in slower assembly processes and monolayers with slightly different characteristics.
Materials for SAMs – Solutions
In situ deprotection of thioacetates affords a solution to some of the current challenges. Thioacetates can be prepared in high purities, and remain stable for extended periods of time. They can be deprotected using relatively mild conditions to generate high purity thiols. These thiols can then begin to assemble immediately, forming highly-organized self-assembled monolayers.
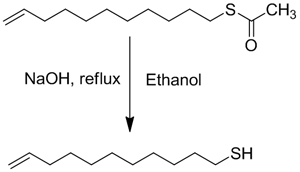
Typical Deprotection Procedure
The following deprotection procedure was used to obtain 11-Mercapto-1-undecene in 86% yield. The thiol was then passed through a silica gel pad to provide high purity (>99%) materials for SAMs.
- In a 250 ml, 3-necked RB flask provided with a reflux condenser and thermometer, dissolve (2 g, 8.76 mmol) S-(10-Undecenyl) thioacetate in 20 ml ethanol under nitrogen.
- Add NaOH (1 g, 25.7 mmol) in 10 mL of water drop-wise to the reaction flask followed by reflux for 2 hrs.
- Bring the reaction mixture to room temperature and neutralize with 5 mL degassed 2M HCl solution.
- Transfer solution to a separatory funnel under inert atmosphere, add 20 mL degassed diethyl ether & 10 mL degassed water. Separate organic layer & wash with 50 ml degassed water.
- Dry the combined organic layer over Na2SO4 and evaporate off the solvent at 40 °C using a rotary evaporator.
To continue reading please sign in or create an account.
Don't Have An Account?