104051
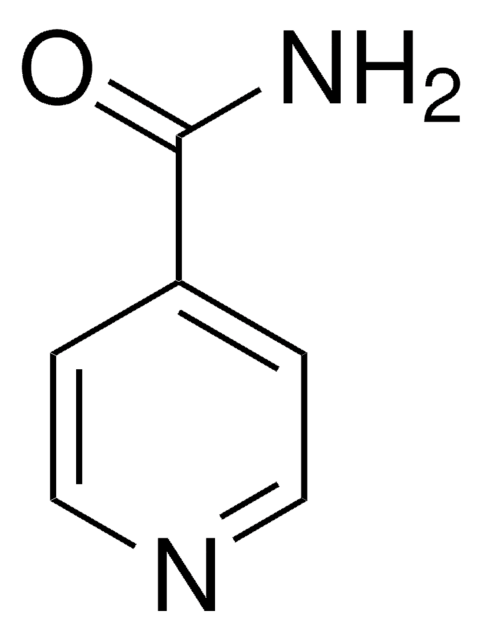
Picolinamide
98%
Synonym(s):
2-Pyridinecarboxamide
Sign Into View Organizational & Contract Pricing
About This Item
Empirical Formula (Hill Notation):
C6H6N2O
CAS Number:
Molecular Weight:
122.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Pricing and availability is not currently available. Contact Technical Service
Recommended Products
Quality Level
Assay
98%
mp
110 °C (dec.) (lit.)
functional group
amide
SMILES string
NC(=O)c1ccccn1
InChI
1S/C6H6N2O/c7-6(9)5-3-1-2-4-8-5/h1-4H,(H2,7,9)
InChI key
IBBMAWULFFBRKK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
1 of 4
This Item | SML0345 | SML0494 | SML1088 |
|---|---|---|---|
| assay 98% | assay ≥98% (HPLC) | assay ≥98% (HPLC) | assay ≥98% (HPLC) |
| Quality Level 100 | Quality Level 100 | Quality Level 100 | Quality Level 100 |
| mp 110 °C (dec.) (lit.) | mp - | mp - | mp - |
| functional group amide | functional group - | functional group - | functional group - |
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protection by picolinamide, a novel inhibitor of poly (ADP-ribose) synthetase, against both streptozotocin-induced depression of proinsulin synthesis and reduction of NAD content in pancreatic islets.
H Yamamoto et al.
Biochemical and biophysical research communications, 95(1), 474-481 (1980-07-16)
Lanthanide complexes with picolinamide.
Condorelli G, et al.
J. Inorg. Nucl. Chem., 36(12), 3763-3766 (1974)
Ángel Manu Martínez et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 23(48), 11669-11676 (2017-06-22)
A practical picolinamide-directed C-H functionalization/alkyne annulation of benzylamine derivatives enabling access to the previously elusive 1,4-dihydroisoquinoline skeleton was developed using molecular O
Study properties of molecular imprinting polymer using a computational approach.
Wu L, et al.
Analyst, 128(7), 944-949 (2003)
Jason L Atkins et al.
Journal of the American Chemical Society, 134(33), 13546-13549 (2012-08-07)
Tris-arenes based on either isophthalic acid or 2,6-dipicolinic acid have been known for more than a decade to bind anions. Recent studies have also demonstrated their ability to transport various ions through membranes. In this report, we demonstrate two important
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service