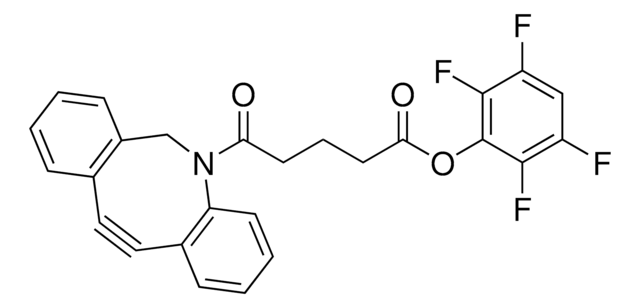
761524
Dibenzocyclooctyne-N-hydroxysuccinimidyl ester
for Copper-free Click Chemistry
Synonym(s):
DBCO-NHS ester, DBCO-SE
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C23H18N2O5
Molecular Weight:
402.40
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
form
solid
reaction suitability
reaction type: click chemistry
reagent type: cross-linking reagent
mp
149-157 (decomposition)
functional group
NHS ester
storage temp.
−20°C
SMILES string
O=C(CCC(ON(C(CC1)=O)C1=O)=O)N2CC3=C(C=CC=C3)C#CC4=C2C=CC=C4
InChI
1S/C23H18N2O5/c26-20(13-14-23(29)30-25-21(27)11-12-22(25)28)24-15-18-7-2-1-5-16(18)9-10-17-6-3-4-8-19(17)24/h1-8H,11-15H2
InChI key
XCEBOJWFQSQZKR-UHFFFAOYSA-N
General description
DBCO-NHS ester is commonly used in bioconjugation. It consists of an NHS ester that reacts with primary amines on biomolecules and an alkyne group that allows for a specific reaction with molecules containing azides. This reaction is carried out through a copper-free click chemistry process called strain-promoted alkyne-azide cycloaddition.
Application
Dibenzocyclooctyne-N-hydroxysuccinimidyl ester is commonly used:
- In the modification and labeling of biomolecules such as antibodies and streptavidin by introducing DBCO groups onto their surfaces
- As a cross linker to conjugate peptide antigens onto the surface of poly(lactic-co-glycolic acid) (PLGA) nanoparticles
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyl N-succinimidyl carbonate for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/969/022/d6776082-2f7a-47c7-bcd4-3830dac0fb7d/640/d6776082-2f7a-47c7-bcd4-3830dac0fb7d.png)


![(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethanol for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/171/632/0556139a-2db5-4678-a6ec-a26a693fd574/640/0556139a-2db5-4678-a6ec-a26a693fd574.png)