PHR1554
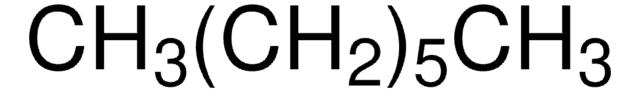
Heptane
Pharmaceutical Secondary Standard; Certified Reference Material
Synonym(s):
Heptane
Select a Size
About This Item
Recommended Products
grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to USP 1304209
vapor density
3.5 (vs air)
vapor pressure
40 mmHg ( 20 °C)
83 mmHg ( 37.7 °C)
CofA
current certificate can be downloaded
autoignition temp.
433 °F
expl. lim.
7 %
packaging
ampule of 3x1.2 mL each
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
refractive index
n20/D 1.387 (lit.)
bp
98 °C (lit.)
mp
−91 °C (lit.)
density
0.684 g/mL at 25 °C (lit.)
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-30°C
SMILES string
CCCCCCC
InChI
1S/C7H16/c1-3-5-7-6-4-2/h3-7H2,1-2H3
InChI key
IMNFDUFMRHMDMM-UHFFFAOYSA-N
General description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Application
Analysis Note
Other Notes
Footnote
Recommended products
1 of 4
This Item | RTC000091 | 1304209 | HX0078 |
|---|---|---|---|
| technique(s) HPLC: suitable, gas chromatography (GC): suitable | technique(s) - | technique(s) - | technique(s) HPLC: suitable |
| application(s) pharmaceutical (small molecule) | application(s) pharmaceutical small molecule | application(s) pharmaceutical (small molecule) | application(s) general analytical |
| format neat | format - | format neat | format - |
| Quality Level 300 | Quality Level 300 | Quality Level - | Quality Level 100 |
| grade certified reference material, pharmaceutical secondary standard | grade certified reference material, pharmaceutical secondary standard | grade pharmaceutical primary standard | grade HPLC grade |
| storage temp. 2-30°C | storage temp. 2-30°C | storage temp. - | storage temp. room temp |
Still not finding the right product?
Explore all of our products under Heptane
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Asp. Tox. 1 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
24.8 °F - closed cup
Flash Point(C)
-4 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.