D1285
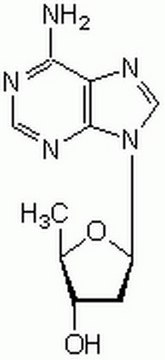
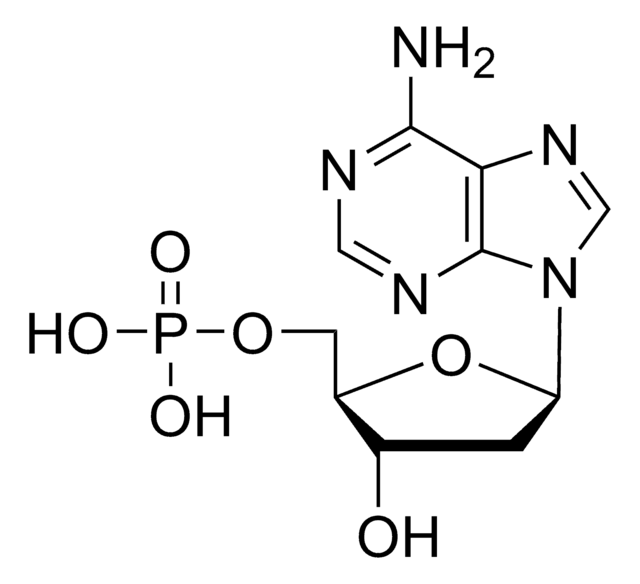
2′,3′-Dideoxyadenosine
≥97% (HPLC)
Synonym(s):
Dideoxyadenosine, ddA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H13N5O2
CAS Number:
Molecular Weight:
235.24
Beilstein:
619924
EC Number:
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
Quality Level
Assay
≥97% (HPLC)
form
powder
optical activity
[α]/D -28.0±1.0
mp
181-184 °C (lit.)
solubility
water: 100 mg/mL, clear, colorless
storage temp.
2-8°C
SMILES string
Nc1ncnc2n(cnc12)[C@H]3CC[C@@H](CO)O3
InChI
1S/C10H13N5O2/c11-9-8-10(13-4-12-9)15(5-14-8)7-2-1-6(3-16)17-7/h4-7,16H,1-3H2,(H2,11,12,13)/t6-,7+/m0/s1
InChI key
WVXRAFOPTSTNLL-NKWVEPMBSA-N
Gene Information
human ... DCK(1633)
Looking for similar products? Visit Product Comparison Guide
General description
2′,3′-Dideoxyadenosine is a nucleoside analog of deoxyadenosine. On deamination, it is converted to dideoxyinosine. It inhibits adenylyl cyclase and may play a key role in the inhibition of tumor progression. It is a potent inhibitor of reverse transcriptase enzyme of human immunodeficiency virus.
Application
2′,3′-Dideoxyadenosine (ddA), a specific adenylyl cyclase inhibitor, is useful in biological process and pathway studies involving adenylyl cyclase activity and cAMP pool modulation.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service