D4943
Dipeptidyl Peptidase IV human
recombinant, expressed in baculovirus infected Sf9 cells, pkg of ≥1.0 units/vial, ≥10 units/mg protein
Synonym(s):
CD26, DPPIV, Dipeptidyl aminopeptidase IV, Glycoprotein GP110
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
recombinant
expressed in baculovirus infected Sf9 cells
Quality Level
form
solution
specific activity
≥10 units/mg protein
mol wt
105 kDa
packaging
pkg of ≥1.0 units/vial
UniProt accession no.
shipped in
wet ice
storage temp.
−20°C
Gene Information
human ... DPP4(1803)
Looking for similar products? Visit Product Comparison Guide
General description
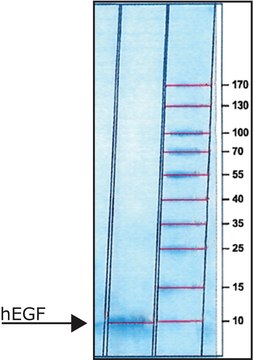
C-terminal histidine-tagged. Soluble form (residues 29-766) MW 105 kDa
Application
Human dipeptidyl peptidase IV has been used to study interactive hemodynamic effects of its inhibition and angiotensin-converting enzyme inhibition in humans. Human dipeptidyl peptidase IV has also been used in a study that informed the understanding of Hymenoptera venom allergies.
The enzyme from Sigma has been used to study the LC-MS (liquid chromatography-mass spectrometry) based assay method for DPP-IV inhibitor screening and substrate discovery.
Biochem/physiol Actions
DPPIV has a post-proline dipeptidyl aminopeptidase activity that hydrolyzes N-terminal dipeptides from the unsubstituted N-terminus of peptides with the sequence of X-Pro-Z and X-Ala-Z. The optimum pH is found to be 7.4-8.7. DPPIV is involved in the regulation of several important physiological processes such as immune functions, inflammation, CNS, endocrine functions, bone marrow mobilization, cancer growth, cell adhesion, glucose hemostasis and sepsis/severe infection.
DPPIV has a post-proline dipeptidyl aminopeptidase activity that hydrolyzes N-terminal dipeptides from the unsubstituted N-terminus of peptides with the sequence of X-Pro-Z and X-Ala-Z. Where X is a nonspecific residue at the N terminus and Z cannot be proline or hydroxyproline.
Native DPPIV is a ubiquitous type II transmembrane glycoprotein and a serine protease of the S9 prolyl-oligopeptidase family. In vivo, it is synthesized with a signal peptide, which functions as the membrane anchoring domain. There is an 88% sequence homology between the human and porcine kidney enzymes. Both exist as homodimers with a subunit molecular weight of ~30 kDa. The high mannose 100 kDa DPPIV precursor is processed in the Golgi to yield a 124 kDa heavily N-and O-linked mature glycoprotein. It is then sorted to the apical membrane through the concerted action of both N- and O-linked glycans and its association with lipid microdomains. The porcine enzyme contains 18.3% carbohydrates, which the glycan composition is 0.9% fucose, 3.4% mannose, 5.1% galactose, 8.2% glucosamine, and 0.7% sialic acid. DPPIV is highly expressed on endothelial cells, epithelial cells, and lymphocytes. It is also present in plasma in its soluble form.
Unit Definition
One unit will produce 1.0 μmole of p-nitroaniline from Gly-L-Pro p-nitroanilide per min in 100 mM Tris-HCl at pH 7.6 at 37 °C.
Physical form
Supplied as a solution in 10 mM Tris-HCl, pH 7.6, 200 mM NaCl, 1 mM EDTA and 10% glycerol.
Other Notes
View more information on Dipeptidyl Peptidase IV at www.sigma-aldrich.com/enzymeexplorer.
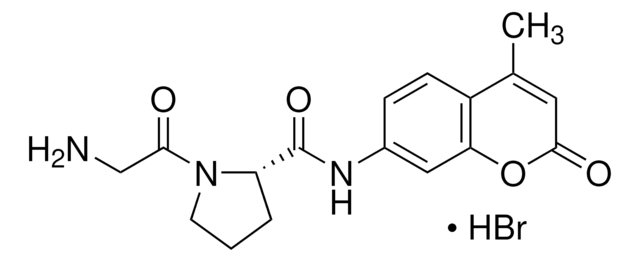
inhibitor
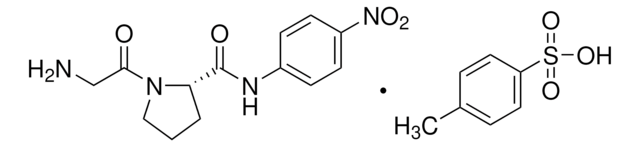
substrate
Product No.
Description
Pricing
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service