S1318
STO-609-acetic acid
≥95% (HPLC), solid
Synonym(s):
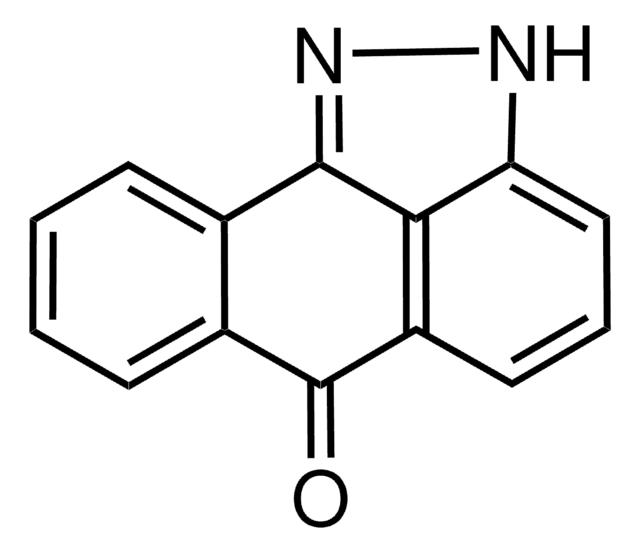
7-Oxo-7H-benzimidazo[2,1-a]benz[de]isoquinoline-3-carboxylic acid - acetic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C19H10N2O3 · C2H4O2
CAS Number:
Molecular Weight:
374.35
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥95% (HPLC)
form
solid
mp
>300 °C
solubility
DMSO: 5 mg/mL, clear (warmed)
storage temp.
2-8°C
SMILES string
CC(O)=O.OC(=O)c1ccc2-c3nc4ccccc4n3C(=O)c5cccc1c25
InChI
1S/C19H10N2O3.C2H4O2/c22-18-13-5-3-4-10-11(19(23)24)8-9-12(16(10)13)17-20-14-6-1-2-7-15(14)21(17)18;1-2(3)4/h1-9H,(H,23,24);1H3,(H,3,4)
InChI key
WNRSTFUVBWNELX-UHFFFAOYSA-N
General description
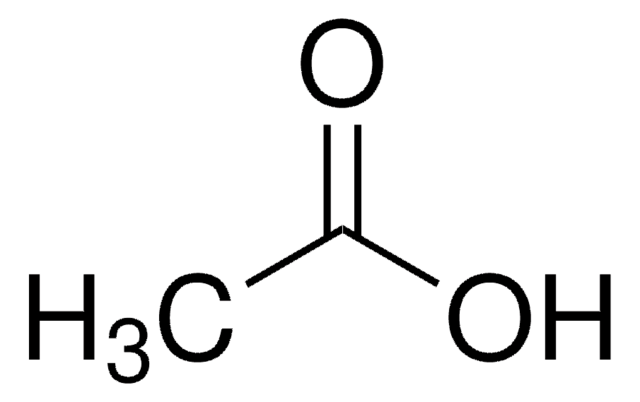
1:1 adduct with acetic acid
STO-609 reduces tumorigenicity of liver cancer cells in vivo. TO-609 reduces the activation of AMP (adenosine monophosphate)-activated protein kinase (AMPK) by ionomycin in a dose dependant manner.
Application
STO609 has been used as a calcium/calmodulin-dependent protein kinase kinase (CAMKK) inhibitor in mouse MLL-AF9 acute myeloid leukemia (AML) cells.
Biochem/physiol Actions
Selective Ca2+/Calmodulin-dependent protein kinase kinase (CaM-KK) antagonist.
Selective Ca2+/Calmodulin-dependent protein kinase kinase (CaM-KK) antagonist. Inhibits CamKKa and CaMKKb with Ki = 80 and 15 ng/mL, respectively. Does not inhibit downstream CaM kinases (CaMKI and CaMKIV).
Features and Benefits
This compound is featured on the Ca/CaMKs page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service