T4299
Trypsin-EDTA solution
1 ×, sterile-filtered, BioReagent, suitable for cell culture, 500 BAEE units porcine trypsin and 180 μg EDTA, 4Na per ml in Dulbecco′s PBS without calcium and magnesium
Synonym(s):
Cocoonase, Tryptar, Tryptase
About This Item
Recommended Products
biological source
Porcine pancreas
sterility
sterile-filtered
product line
BioReagent
form
solution
concentration
1 ×
technique(s)
cell culture | mammalian: suitable
impurities
Porcine parvovirus, none detected (9 CFR)
pH
7.0-7.6
shipped in
dry ice
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
1 of 4
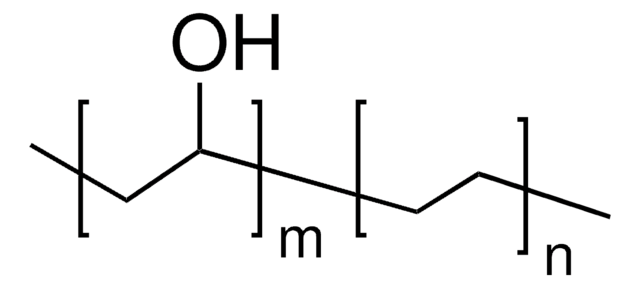
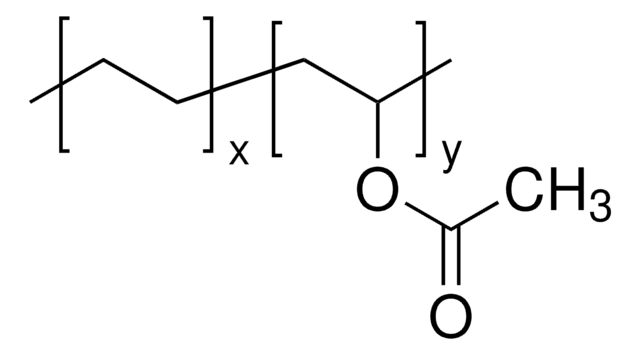
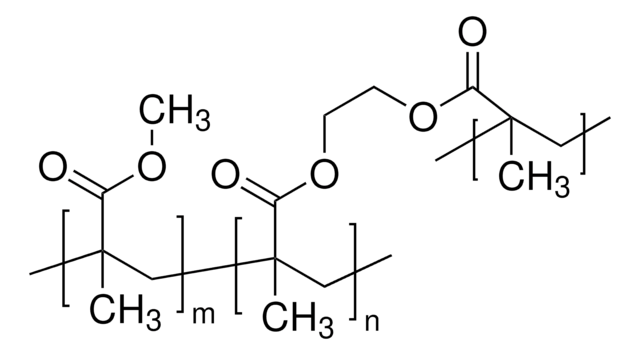
This Item | 340502 | 190845 | 363146 |
|---|---|---|---|
| form pellets | form beads | form powder | form powder or crystals |
| density 1.19 g/mL at 25 °C | density - | density 1.27 g/mL at 25 °C (lit.) | density - |
| melt index 3.8 g/10 min (210°C) | melt index (41-63 dg/min (190°C/2.16kg)) | melt index - | melt index - |
| composition ethylene, 32 mol % | composition vinyl acetate, 40 wt. % | composition - | composition - |
| hardness 100 (Rockwell M, ASTM D 785) | hardness 40 (Shore A-2, ASTM D 2240) | hardness - | hardness - |
| impurities <0.7% polymerized vinyl acetate | impurities - | impurities - | impurities - |
Application
Biochem/physiol Actions
Serine protease inhibitors, including DFP, TLCK, APMSF, AEBSEF, and aprotinin, amongst others, will inhibit Trypsin.
Components
Caution
related product
Storage Class Code
12 - Non Combustible Liquids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service