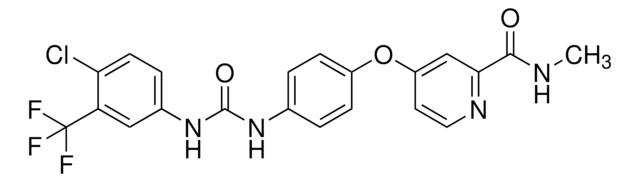
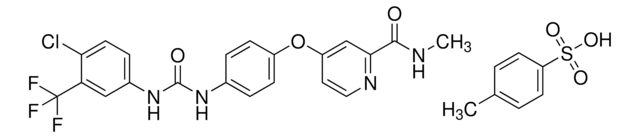
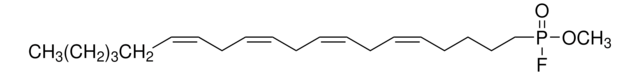
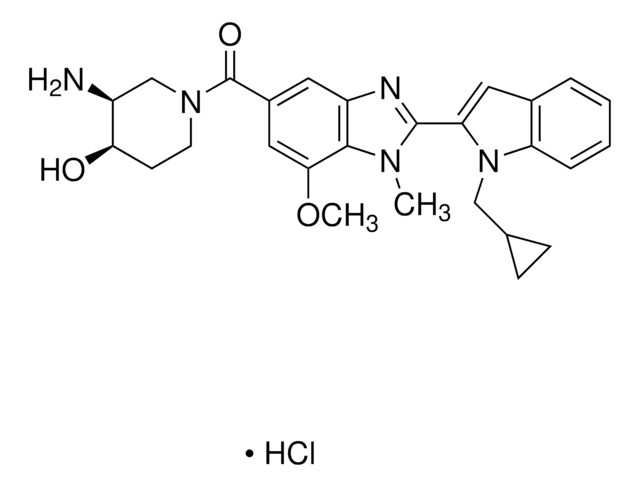
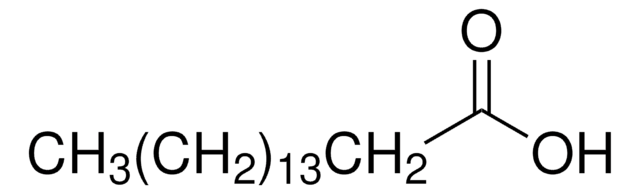T6575
TOFA
≥98% (HPLC)
Synonym(s):
5-(Tetradecyloxy)-2-furoic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C19H32O4
CAS Number:
Molecular Weight:
324.45
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Assay
≥98% (HPLC)
form
powder
color
white to beige
mp
112-115 °C
solubility
DMSO: 2 mg/mL, clear
storage temp.
−20°C
SMILES string
CCCCCCCCCCCCCCOc1ccc(o1)C(O)=O
InChI
1S/C19H32O4/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-22-18-15-14-17(23-18)19(20)21/h14-15H,2-13,16H2,1H3,(H,20,21)
InChI key
CZRCFAOMWRAFIC-UHFFFAOYSA-N
Application
TOFA has been used as a lipid biosynthesis inhibitor in mesenchymal stromal cells (MSCs), human pluripotent stem cells., an acetyl-CoA carboxylase 1 inhibitor in murine adipocyte cell lines., a lipolysis inhibitor in cancer stem cells (CSCs).
TOFA has been used as an ACC inhibitor to study its effect on insulin secretion in INS-1E cells.
Biochem/physiol Actions
5-(Tetradecyloxy)-2-furoic acid (TOFA) elicits hypolipidemic functionality by favoring fatty acid breakdown and at the same time preventing biosynthesis. It induces apoptosis in pancreatic cancer cells and favors tumor suppression.
Studies have reported that TOFA can inhibit the anorectic effect of subcutaneous tamoxifen (TMX) on food intake during refeeding experiments in rats.
TOFA is a cell-permeable, potent, reversible, and competitive inhibitor of acetyl-CoA carboxylase (ACC).
TOFA is a cell-permeable, potent, reversible, and competitive inhibitor of acetyl-CoA carboxylase (ACC). TOFA is a key enzyme involved in fatty acid biosynthesis. TOFA inhibits cellular fatty acid synthesis in a dose-dependent manner (IC50 = 4 μM in human breast cancer cell line MCF7).
Preparation Note
TOFA is soluble in DMSO at a concentration that is greater than 2 mg/ml.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service