1466674
USP
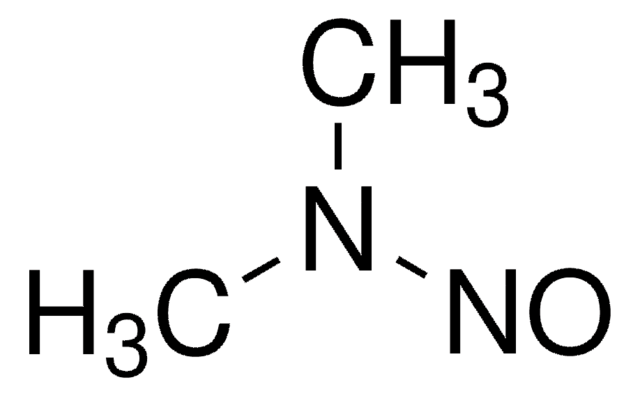
N-Nitrosodimethylamine (NDMA)
United States Pharmacopeia (USP) Reference Standard
Synonym(s):
N-Methyl-N-nitrosomethanamine, NDMA
About This Item
Recommended Products
form
liquid
packaging
pkg of 1 mg
manufacturer/tradename
USP
application(s)
pharmaceutical (small molecules)
format
single component solution (solution in methanol)
storage temp.
−20°C
SMILES string
N(N=O)(C)C
InChI
1S/C2H6N2O/c1-4(2)3-5/h1-2H3
InChI key
UMFJAHHVKNCGLG-UHFFFAOYSA-N
General description
N-Nitrosodimethylamine is another carcinogenic compound, with particular focus placed on its detection and control in biologic and pharmaceutical products. Like NDEA, NDMA can form as a by-product during manufacturing processes. Regulatory agencies require its rigorous monitoring, and it plays a key role in ensuring the purity and safety of biologic products.
The United States Pharmacopeia (USP) provides quality standards for biologics to ensure their safety, efficacy, and quality throughout the manufacturing process. These standards assist manufacturers in adhering to regulatory requirements and help safeguard public health by reducing risks associated with biologics.
Application
It is also used to prepare standard, standard stock, nitrosamine RS stock, nitrosamine standards stock solution mixture, and sensitivity stock solutions to determine NDMA impurity in drug substances and drug products(valsartan, irbesartan, and losartan potassium etc.) by chromatography method according to the general chapter 〈1469〉 of United States Pharmacopeia.
Analysis Note
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Carc. 1B - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes,Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
49.5 °F
Flash Point(C)
9.7 °C
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service