1466674
USP
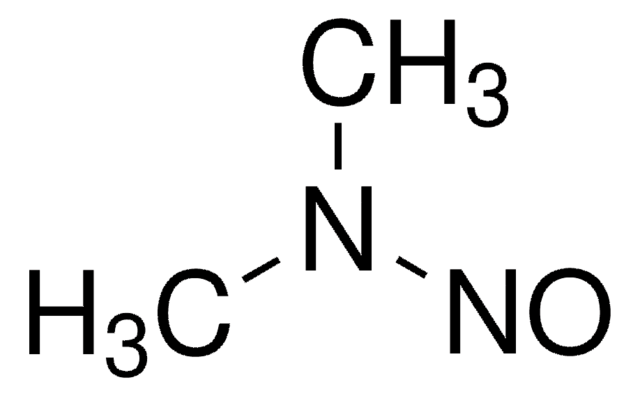
N-Nitrosodimethylamine (NDMA)
United States Pharmacopeia (USP) Reference Standard
Synonyme(s) :
N-Methyl-N-nitrosomethanamine, NDMA
About This Item
Produits recommandés
Forme
liquid
Conditionnement
pkg of 1 mg
Fabricant/nom de marque
USP
Application(s)
pharmaceutical (small molecules)
Format
single component solution (solution in methanol)
Température de stockage
−20°C
Chaîne SMILES
N(N=O)(C)C
InChI
1S/C2H6N2O/c1-4(2)3-5/h1-2H3
Clé InChI
UMFJAHHVKNCGLG-UHFFFAOYSA-N
Description générale
N-Nitrosodimethylamine is another carcinogenic compound, with particular focus placed on its detection and control in biologic and pharmaceutical products. Like NDEA, NDMA can form as a by-product during manufacturing processes. Regulatory agencies require its rigorous monitoring, and it plays a key role in ensuring the purity and safety of biologic products.
The United States Pharmacopeia (USP) provides quality standards for biologics to ensure their safety, efficacy, and quality throughout the manufacturing process. These standards assist manufacturers in adhering to regulatory requirements and help safeguard public health by reducing risks associated with biologics.
Application
It is also used to prepare standard, standard stock, nitrosamine RS stock, nitrosamine standards stock solution mixture, and sensitivity stock solutions to determine NDMA impurity in drug substances and drug products(valsartan, irbesartan, and losartan potassium etc.) by chromatography method according to the general chapter 〈1469〉 of United States Pharmacopeia.
Remarque sur l'analyse
Autres remarques
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Carc. 1B - Flam. Liq. 2 - STOT SE 1
Organes cibles
Eyes,Central nervous system
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
49.5 °F
Point d'éclair (°C)
9.7 °C
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique