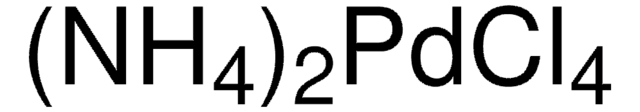205796
Potassium tetrachloropalladate(II)
98%
Synonym(s):
Potassium palladium(II) chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
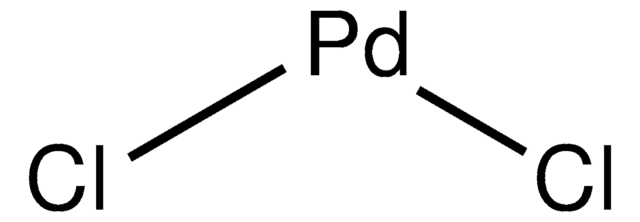
K2PdCl4
CAS Number:
Molecular Weight:
326.43
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
form:
crystals
Recommended Products
Quality Level
Assay
98%
form
crystals
reaction suitability
core: potassium
mp
105 °C (dec.) (lit.)
density
2.67 g/mL at 25 °C (lit.)
SMILES string
[K+].[K+].Cl[Pd--](Cl)(Cl)Cl
InChI
1S/4ClH.2K.Pd/h4*1H;;;/q;;;;2*+1;+2/p-4
InChI key
LGCKLDWLSVFMGL-UHFFFAOYSA-J
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Potassium tetrachloropalladate(II) is a dark brown crystalline solid widely used as a Pd source in the field of catalysis, nanomaterial synthesis, and electronics.
Application
Potassium tetrachloropalladate(II) can be used:
- As a precursor to synthesize Pd nanoparticles for catalytic degradation of organic pollutants and Pd-GO electrocatalyst in formic acid and ethanol oxidation.
- To synthesize Pd–Pt alloy nanocrystals (NCs) with hollow structures by a galvanic replacement method with uniform Pd octahedral and cubic NCs as sacrificial templates. The hollow NCs exhibited higher ORR activities.
- To synthesize immobilized Pd catalysts, a versatile method involves the layer-by-layer deposition of PAA and PEI-Pd(II) on alumina, followed by the reduction of Pd2+. This approach offers several benefits, including the stabilization of particles through the polyelectrolyte matrix, introduction of selectivity, and a significant reduction in undesired isomerization. Expanding the application of polyelectrolyte films holds promise for further enhancing selectivity in hydrogenation and other reactions.
- To fabricate conductive and porous metal-organic frameworks(MOFs) for gas sensing applications and also to synthesize bimetallic Pd/SnO2 nanoparticles on metal organic framework (MOF) as an electrocatalyst for ethanol oxidation.
- To prepare rigid macrocyclic pincer catalysts possessing polyaromatic ligands with enhanced catalytic activity.
Features and Benefits
Used in the synthesis of semiconducting metal-containing polymers in which the polypyrrole backbone has a conformational energy minimum and is nearly planar. Reacts with bis(dithiolates) to metal-bis(dithiolates) with applications in laser Q-switch materials, optical CD recording media, bar code material and superconductivity.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service