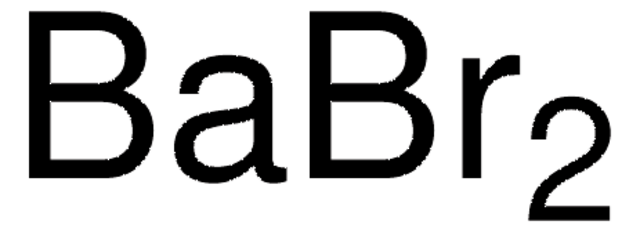342920
Barium chloride
99.9% trace metals basis
Synonym(s):
Dichlorobarium
About This Item
Recommended Products
Quality Level
Assay
99.9% trace metals basis
form
powder
impurities
≤1500.0 ppm Trace Metal Analysis
mp
963 °C (lit.)
density
3.856 g/mL at 25 °C (lit.)
SMILES string
Cl[Ba]Cl
InChI
1S/Ba.2ClH/h;2*1H/q+2;;/p-2
InChI key
WDIHJSXYQDMJHN-UHFFFAOYSA-L
General description
1 of 4
This Item | Z291641 | Z291633 | Z291994 |
|---|---|---|---|
| material amber glass vial | material amber glass vial (standard opening) | material clear glass vial (standard opening) | material clear glass vial, low-density polyethylene closure |
| packaging pkg of 100 | packaging pkg of 100 ea | packaging pkg of 100 ea | packaging pkg of 250 ea |
| feature closure type crimp top vial | feature closure type screw top vial | feature closure type screw top vial | feature closure type polyethylene cap |
| O.D. × H × I.D. 12 mm × 32 mm × 4.6 mm | O.D. × H × I.D. 12 mm × 32 mm × 4.6 mm | O.D. × H × I.D. 12 mm × 32 mm × 4.6 mm | O.D. × H × I.D. - |
| volume 2 mL | volume 2 mL | volume 2 mL | volume 1 mL |
| fitting thread for 11 mm | fitting thread for 8-425 | fitting thread for 8-425 | fitting plug for 8 mm |
Still not finding the right product?
Explore all of our products under Barium chloride
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Acute Tox. 4 Inhalation - Eye Irrit. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.