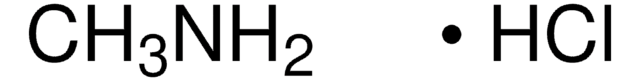GF178
C1 Inhibitor
Synonym(s):
C1 Inh, C1 esterase inhibitor, Serpin G1
Sign Into View Organizational & Contract Pricing
About This Item
UNSPSC Code:
12352200
eCl@ss:
32160405
NACRES:
NA.77
Pricing and availability is not currently available.
Recommended Products
1 of 3
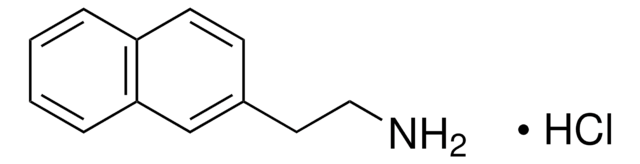
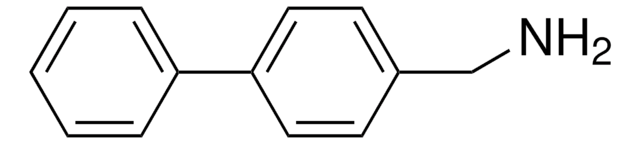
This Item | M0505 | 399655 |
|---|---|---|
| assay 95% | assay ≥98% | assay 99% |
| Quality Level 200 | Quality Level 200 | Quality Level 100 |
| form powder | form powder or crystals | form - |
| mp 258 °C (dec.) (lit.) | mp 231-233 °C (lit.) | mp 80-83 °C (lit.) |
| functional group amine | functional group - | functional group hydroxyl |
General description
C1 Inhibitor is a member of the serpin family of structurally related proteins, and is the primary regulator of the immune complement system. C1 Inhibitor is a protease inhibitor that functions to inhibit the complement system in order to prevent over-activation or spontaneous activation. Inhibition is achieved by binding to and irreversibly inhibiting the C1r and C1s proteases of the C1 complex, which has the effect of shutting down all subsequent downstream events in the complement activation cascade. C1 inhibitor can also inhibit various other proteases, including Kallikrein, Factor XIa, and Factor XIIa.
Recombinant Human C1 Inhibitor is a highly glycosylated glycoprotein containing 445 amino acid residues (49.4kDa), corresponding to amino acids 56 – 500 of the C1 inhibitor precursor, and is fully functional in its ability to inhibit the C1 complex.
Recombinant Human C1 Inhibitor is a highly glycosylated glycoprotein containing 445 amino acid residues (49.4kDa), corresponding to amino acids 56 – 500 of the C1 inhibitor precursor, and is fully functional in its ability to inhibit the C1 complex.
Product Source: Protein is expressed in E.coli.
Application
Research Category
Stem Cell Research
Stem Cell Research
Research Sub Category
Growth Factors & Receptors
Growth Factors & Receptors
Quality
Measured by its ability to inhibit recombinant human complement component C1a cleavage of a colorimetric peptide substrate, N Carbobenzyloxy-Lys- ThioBenzyl ester (Z-K-SBzl). The expected IC50 is ≤ 2.6 nM.
Physical form
Product is presented in 10mM Sodium Phosphate, pH 7.5 and is filtered through a 0.2 micron filter before lyophilization.
Storage and Stability
Store at -20°C for up to 4 months from date of receipt Centrifuge the vial prior to opening.
Reconstitute in water to a concentration of 0.1-1.0 mg/ml. Do not vortex. For extended storage, it is recommended to further dilute in a buffer containing a carrier protein and store in working aliquots at -20°C.
Reconstitute in water to a concentration of 0.1-1.0 mg/ml. Do not vortex. For extended storage, it is recommended to further dilute in a buffer containing a carrier protein and store in working aliquots at -20°C.
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fabien Giroud et al.
Biosensors & bioelectronics, 87, 957-963 (2016-09-26)
We report the functionalization of multi-walled carbon nanotubes (MWCNTs) electrodes by a bifunctional nitroaromatic molecule accomplished via π-π interactions of a pyrene derivative. DTNB (5,5'-dithiobis(2-nitrobenzoic acid)) has the particularity to possess both electroactivable nitro groups and negatively charged carboxylic groups.
Sudarat Yenjai et al.
Journal of photochemistry and photobiology. B, Biology, 173, 35-42 (2017-05-30)
A new photochemical reagent, succinic acid-1(1-pyrene)methylamide (PMA-SUC), was developed to recognize the specific binding sites on model proteins, egg-white lysozyme and avidin. The interaction of the photochemical reagent with the proteins was studied by UV-Vis, fluorescence spectroscopic methods and docking
Synthesis and fluorescence properties of carbazole based asymmetric functionalized star shaped polymer.
Guzel M, et al.
Journal of the Electrochemical Society, 164(2), H49-H55 (2017)
Reactively formed block and graft copolymers as compatibilizers for polyamide 66/PS blends.
Jeon HK, et al.
Polymer, 45(1), 197-206 (2004)
Intramolecular excimer formation and sensing behavior of new fluorimetric probes and their interactions with metal cations and barbituric acids.
Lodeiro C, et al.
Sensors and Actuators B, Chemical, 115(1), 276-286 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service