C4879
α-Chymotrypsinogen A from bovine pancreas
essentially salt-free, lyophilized powder
Synonym(s):
chymotrypsin A zymogen
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Recommended Products
biological source
bovine pancreas
Quality Level
type
Type II
form
essentially salt-free, lyophilized powder
specific activity
≥40 units/mg solid
mol wt
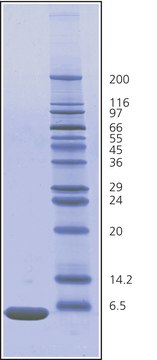
25,656 Da by calculation
purified by
6× crystallization
solubility
1 mM HCl: soluble 10 mg/mL, clear, colorless
UniProt accession no.
foreign activity
α-chymotrypsin ≤1 U/mg (prior to activation by trypsin)
storage temp.
−20°C
Gene Information
cow ... CTRB1(618826)
Related Categories
General description
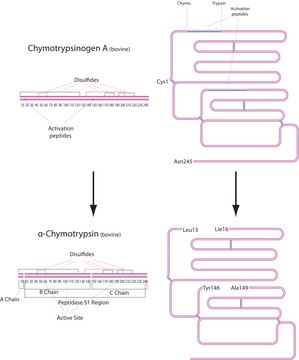
Chymotrypsinogen from bovine pancreas is a zymogen containing 5 disulfide bridges. It has an isoelectric pH of 8.97.
Application
α-Chymotrypsinogen A from bovine pancreas has been used as model protein crystallization reproducibility studies. It has also been used in the hydrolysis of α-gliadins prior to mass spectroscopy studies.
The enzyme from Sigma has been used in the non-invasive determination of solid-state protein conformation using near infrared (NIR) spectroscopy. It has been used to study the partitioning of protein in polymer/polymer aqueous two-phase systems. The enzyme has also been used for self-interaction chromatography applications, to test the rapid measurement of protein osmotic second virial coefficients. In this technique, the protein is immobilized on chromatographic particles and its retention is measured using isocratic elution.
Biochem/physiol Actions
A serine protease that hydrolyzes peptide bonds with aromatic or large hydrophobic side chains (Tyr, Trp, Phe, Met) on the carboxyl end of the peptide bond.
Chymotrypsinogen A requires limited proteolysis for its activation. Chymotrypsinogen A may be activated by trypsin and chymotrypsin (autolytic activation) to form m α, β, γ, δ and π chymotrypsin (depending upon the conditions of activation). Chymotrypsin is a protease that will preferentially cleave peptides on the carboxyl side of aromatic amino acids including tryptophan, tyrosine, and phenylalanine. It will also hydrolyze peptides on the carboxyl side of leucine, methionine, and alanine.
Unit Definition
After activation to Chymotrypsin, one unit will hydrolyze 1.0 μmole of BTEE per min at pH 7.8 at 25 °C.
Other Notes
View more information on chymotrypsin at www.sigma-aldrich.com/enzymeexplorer
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service