C7661
Rat Collagen Type I
from rat tail, powder, suitable for cell culture
Sign Into View Organizational & Contract Pricing
All Photos(5)
About This Item
Recommended Products
Product Name
Collagen from rat tail, Bornstein and Traub Type I, powder, BioReagent, suitable for cell culture
biological source
rat tail
Quality Level
product line
BioReagent
form
powder
mol wt
120—160 kDa
packaging
glass bottle of 5 mg
technique(s)
cell culture | mammalian: suitable
surface coverage
6‑10 μg/cm2
solubility
soluble
NCBI accession no.
UniProt accession no.
Binding Specificity
Peptide Source: Fibrinogen
Peptide Source: Laminin
storage temp.
2-8°C
Gene Information
rat ... Col1a1(29393)
Looking for similar products? Visit Product Comparison Guide
General description
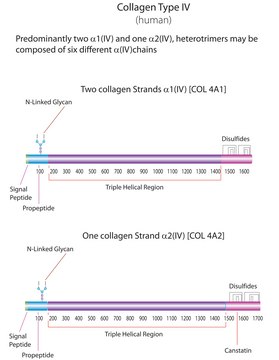
All collagen molecules are composed of three polypeptide chains arranged in a triple helical conformation, with a primary structure that is mostly a repeating motif with glycine in every third position and proline or 4-hydroxyproline frequently preceding the glycine residue. Type I collagen differs from other collagens by its low lysine hydroxylation and low carbohydrate composition. Col1a1 (collagen, type I, α1) or collagen is a major structural human protein, which assembles in the form of fibrils. It is a very long, thin and the most abundant protein found in the human body. It is a supercoiled right-helix of three left-handed polypeptide chains. These chains are composed of ~1040 amino acids, which are essentially repeats of three amino acids -(Gly-X-Y)n. Gly is glycine and X and Y can be any amino acids, but in humans are usually proline and hydroxyproline, respectively.
Product is clear to hazy colorless solution with a few insolubles at 1 mg/ml in water with 2 μl acetic acid (or 0.1 N acetic acid). The insolubles can be removed by settling or centrifugation.
Application
Collagen from rat tail is used for the following applications:
- Immunohistochemistry
- Cellular activity assays
- Used in generation of dorsal root ganglion (DRG) explant cultures
- Used as one of the components during the preparation of the functionalized surface (in NMR setup)
- Used in cell culture (the glass coverslips were coated with nanowires at high concentrations mixed with collagen)
- Used for biofunctionalization of the microchannels
Biochem/physiol Actions
Collagen is an essential ingredient of connective tissue. Studies in a Chinese family show that mutation in COL1A1 (collagenase type I) is linked with type I osteogenesis imperfecta. Collagen is linked with subchondral turnover of bone, and might have potential as marker to determine the state of joint space narrowing and osteophytes in osteoarthritis.Collagen from rat tail is intended to produce thin layer coatings on tissue culture plates to facilitate attachment of anchorage-dependent cells, recommended for use at 6-10 μg/cm2. It is NOT intended for production of 3-D gels. Type I collagen is often used in cell culture as an attachment substratum with myoblasts, spinal ganglia, hepatocytes, embryonic lung, heart explants, fibroblasts, endothelial cells, and islet cells have all been cultured successfully on films or gels of type I collagen. Collagen type I may also be used in research of Idiopathic pulmonary fibrosis (IPF), studies on the effect of ER stress IPF on lung fibroblasts. Collagen in acidic solution can produce three dimensional scaffolding with use in bioengineering and cell culture applications.
Preparation Note
Product is clear to hazy colorless solution with a few insolubles at 1 mg/ml in water with 2 μl acetic acid (or 0.1 N acetic acid). The insolubles can be removed by settling or centrifugation.
Other Notes
Collagen is classified into a number of structurally and genetically distinct types. We use the nomenclature proposed by Bornstein and Traub. Do not confuse Sigma type designations with recognized collagen classification types.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service