I2879
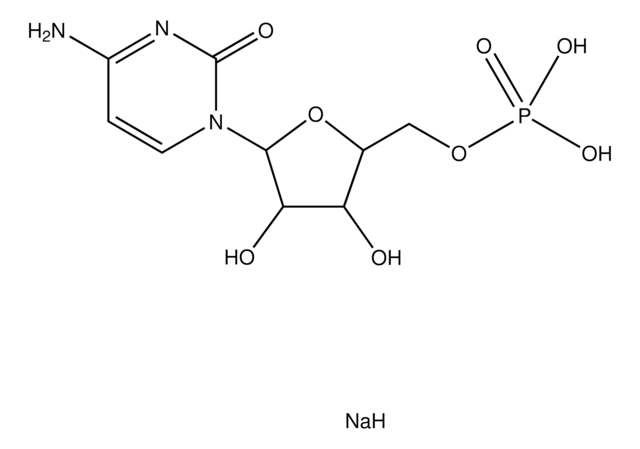
Inosine 5′-monophosphate from Saccharomyces cerevisiae
≥98%
Synonym(s):
I-5′-P, IMP, Inosinic Acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H13N4O8P
CAS Number:
Molecular Weight:
348.21
EC Number:
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
Quality Level
Assay
≥98%
form
powder
solubility
water: 100 mg/mL, clear to slightly hazy, colorless to almost colorless
storage temp.
−20°C
SMILES string
CC1(OCP(O)(O)=O)[C@@](O)(C)[C@@](C)(O)[C@@](N(C=N2)C3=C2C(NC=N3)=O)(C)O1
InChI
1S/C10H13N4O8P/c15-6-4(1-21-23(18,19)20)22-10(7(6)16)14-3-13-5-8(14)11-2-12-9(5)17/h2-4,6-7,10,15-16H,1H2,(H,11,12,17)(H2,18,19,20)
InChI key
GRSZFWQUAKGDAV-UHFFFAOYSA-N
Related Categories
Application
Inosine 5′-monophosphate (IMP) has been used as an umami tastant along with MSG to study their cortical responses and interactions in the human brain. It may be used as a substrate to study the distribution, specificity, and kinetics of inosine-5′-monophosphate dehydrogenase (IMPDH).
Biochem/physiol Actions
Inosine 5′-monophosphate is a purine nucleotide with a flavor-enhancing property. It acts as a precursor for the synthesis of both guanosine monophosphate (GMP) and adenosine monophosphate (AMP).
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service