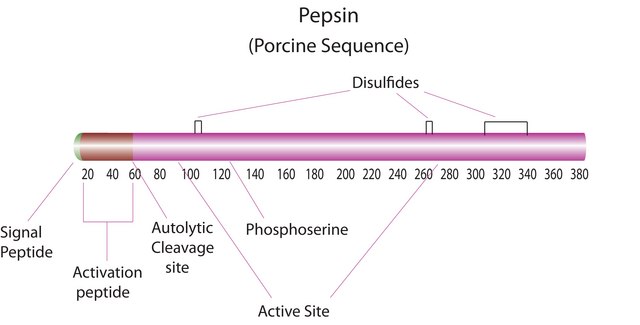
P7125
Pepsin from porcine gastric mucosa
powder, ≥400 units/mg protein
Synonym(s):
Pepsin A, Pepsin from hog stomach
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Recommended Products
biological source
Porcine gastric mucosa
Quality Level
form
powder
specific activity
≥400 units/mg protein
mol wt
35 kDa
solubility
10 mM HCl: soluble 1.0 mg/mL, clear to faintly turbid, colorless
UniProt accession no.
storage temp.
2-8°C
Gene Information
pig ... LOC396892(396892)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
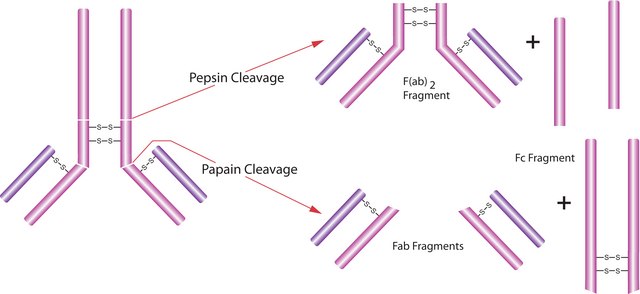
Pepsin cleavage can be used to produce F(ab′)2 fragments of antibodies. pepsin at www.sigma-aldrich.com/enzymeexplorer.
Pepsin is a peptidase used to digest proteins and is commonly used in the preparation of Fab fragments from antibodies. Pepsin, from porcine gastric mucosa, has been used to hydrolyze dry cervical samples in mice. Product P7125 is provided in powder form. Product P7125 has been used to denature DNA from kidney cells and to digest pathology samples from anal canal carcinomas (ACC) biopsies prior to EGFR staining.
The enzyme from Sigma has been used to obtain total vitamin B12 content in food products prior using immunoaffinity columns It has also been used to digest minced soft tissue of snails prior to the isolation of the third stage larvae (L3).
Biochem/physiol Actions
It does not cleave at valine, alanine, or glycine linkages. Z-L-tyrosyl-L-phenylalanine, Z-L-glutamyl-L-tyrosine, or Z-L-methionyl-L-tyrosine may be used as substrates for pepsin digestion. Pepsin is inhibited by several phenylalanine-containing peptides.
Pepsin hydrolyzes peptide bonds, not amide or ester linkages. Pepsin cleaves peptides with an aromatic acid on either side of the peptide bond. Sulfur-containing amino acids increase susceptibility to hydrolysis when they are close to the peptide bond. Pepsin preferentially cleaves at the carboxyl side of phenylalanine and leucine and at the carboxyl side of glutamic acid residues. Cleaves Phe-Val, Gln-His, Glu-Ala, Ala-Leu, Leu-Tyr, Tyr-Leu, Gly-Phe, Phe-Phe and Phe-Tyr bonds in the β chain of insulin
Pepsin is the major proteolytic enzyme produced in the stomach. It digests proteins through the cleavage of interior peptide linkages.
Pepsin is the major proteolytic enzyme produced in the stomach. It digests proteins through the cleavage of interior peptide linkages.
Preferential cleavage: hydrophobic and aromatic residues in P1 and P1′ postitions. Cleaves Phe-Val, Gln-His, Glu-Ala, Ala-Leu, Leu-Tyr, Tyr-Leu, Gly-Phe, Phe-Phe and Phe-Tyr bonds in the β chain of insulin
Unit Definition
One unit will produce a ΔA280 of 0.001 per min at pH 2.0 at 37 °C, measured as TCA-soluble products using hemoglobin as substrate. (Final volume = 16 mL. Light path = 1 cm.)
Analysis Note
Optimum pH is 2-4. Active in 4 M urea and 3 M guanidine HCl. Stable at 60 °C. Pepsin is irreversibly inactivated at pH 8.0 - 8.5.
Protein determined by E1%/280
Other Notes
View more information on pepsin at www.sigma-aldrich.com/enzymeexplorer.
inhibitor
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service