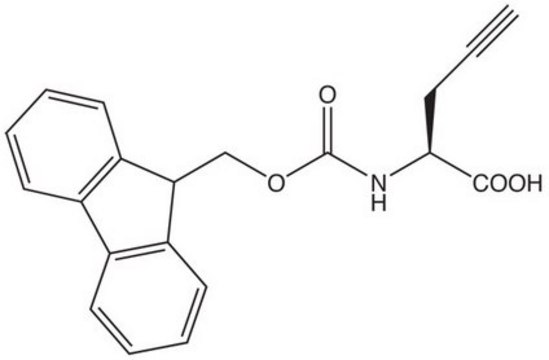
P7888
DL-Propargylglycine
cystathionine γ-lyase inhibitor
Synonym(s):
2-Amino-4-pentynoic acid, PAG
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H7NO2
CAS Number:
Molecular Weight:
113.11
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Quality Level
Assay
≥98% (TLC)
form
powder
color
white
application(s)
cell analysis
storage temp.
−20°C
SMILES string
NC(CC#C)C(O)=O
InChI
1S/C5H7NO2/c1-2-3-4(6)5(7)8/h1,4H,3,6H2,(H,7,8)
InChI key
DGYHPLMPMRKMPD-UHFFFAOYSA-N
Application
- Metabolic Labeling Strategy Boosted Antibacterial Efficiency for Photothermal and Photodynamic Synergistic Bacteria-Infected Wound Therapy.: This study demonstrates the enhanced antibacterial efficiency of a metabolic labeling strategy using ᴅʟ-Propargylglycine in photothermal and photodynamic therapy for treating bacteria-infected wounds (Li et al., 2022).
- Metabolic Labeling of Peptidoglycan with NIR-II Dye Enables In Vivo Imaging of Gut Microbiota.: This research utilizes ᴅʟ-Propargylglycine for metabolic labeling, allowing near-infrared II imaging of gut microbiota in vivo, providing new insights into microbiome studies (Wang et al., 2020).
- Hydrogen sulfide upregulated mRNA expressions of sodium bicarbonate cotransporter1, trefoil factor1 and trefoil factor2 in gastric mucosa in rats.: Investigates the role of ᴅʟ-Propargylglycine, in upregulating specific mRNA expressions in gastric mucosa, with implications for gastrointestinal research (Cheraghi et al., 2016).
Biochem/physiol Actions
DL-Propargylglycine (PAG) is an irreversible inhibitor of the enzyme cystathionine γ-lyase (GCL), a key enzyme involved in glutathione synthesis and the metabolic transsulfuration pathway which regulates homocysteine concentration and mediates cysteine synthesis. Treatment of animal disease models with PAG was shown to alleviate symptoms of diseases that impair the formation or action of hydrogen sulfide (H2S).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service