S6317
L-Sulforaphane
≥95% (HPLC), oil
Synonym(s):
(R)-1-Isothiocyanato-4-(methylsulfinyl)butane, 4-Methylsulfinylbutyl isothiocyanate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H11NOS2
CAS Number:
Molecular Weight:
177.29
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥95% (HPLC)
form
oil
optical activity
[α]/D -70 to -90°, c = 1.0 in chloroform-d
color
light yellow
solubility
DMSO: >5 mg/mL
storage temp.
−20°C
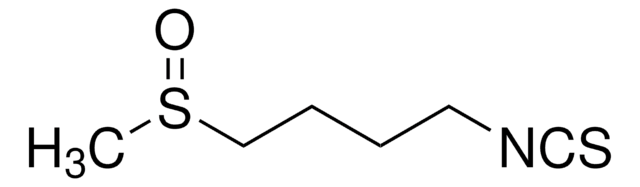
SMILES string
CS(=O)CCCCN=C=S
InChI
1S/C6H11NOS2/c1-10(8)5-3-2-4-7-6-9/h2-5H2,1H3/t10-/m1/s1
InChI key
SUVMJBTUFCVSAD-SNVBAGLBSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
L-Sulforaphane was used to study Nrf2-mediated signaling in mouse primary preadipocytes6 and murine macrophage RAW 264.7 cell line.7
Biochem/physiol Actions
L-Sulforaphane is a potent, selective inducer of phase II detoxification enzymes with anticarcinogenic properties. Organosulfur compound found in cruciferous vegetables, including broccoli.
Sulforaphane is an anti-cancer, anti-microbial and anti-diabetic compound found in cruciferous vegetables.3,4 It induces the production of detoxifying enzymes such as quinone reductase and glutathione S-transferase that cause xenobiotic transformation. Sulforaphane also increases the transcription of tumor suppressor proteins and inhibits histone deacetylases. It modulates inflammatory responses by suppressing the LPS-mediated expression of iNOS, COX-2, IL-1β and TNF-α.3,5
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service