T4455
TEV Protease
Synonym(s):
P1 protease, TEVp, Tobacco Etch Virus protease, rTEV
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
biological source
microbial (Tobacco Etch virus)
Quality Level
recombinant
expressed in E. coli
description
Contains both a histidine tag and a GST tag
form
solution
specific activity
≥3,000 units/mg protein
mol wt
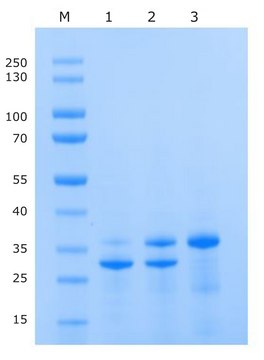
27 kDa
technique(s)
protein purification: suitable
suitability
suitable for purification of HIS tagged recombinant proteins
application(s)
genomic analysis
shipped in
wet ice
storage temp.
−20°C
General description
TEV protease has (NIa) protease catalytic domain which corresponds to a molecular weight of 27 kDa. It is unique with high specificity and is active at low temperature.
The tobacco etch virus (TEV) protease is a useful tool for the removal of fusion tags from recombinant proteins.
Application
TEV Protease has been used in the removal of histidine tag from recombinant mitogen activated protein kinase 14 (MAPK14), Connexin43 (Cx43) c-terminal fragment and δ1-pyrroline-5-carboxylate reductase from Oryza sativa. It has also been used in the elution of purified proteasomes from human leukemia cell lines K562.
Biochem/physiol Actions
Immobilized TEV protease on streptavidin-agarose is an efficient tool for fusion protein cleavage. TEV protease has also been used in a study to investigate proteolytic processing of nlrp1b for inflammasome activity.
TEV protease has a strict 7 amino acid cleavage recognition sequence of Glu-Asn-Leu-Tyr-Phe-Gln↓Gly/Ser.
The tobacco etch virus (TEV) protease undergoes autolysis, but mutants of TEV protease are resistant to autolysis. Bacterial expression of recombinant TEV protease leads to poor yields and less solubility. Use of green fluorescent protein fusion TEV protease overcomes these setbacks and finds application in structural genomics research.
Features and Benefits
Optimally engineered and purified for enhanced stability and high specific activity over a broad temperature range.
Physical properties
A modified 52 kDa TEV protease construct containing both GST and His tags for easy removal using glutathione or His-Select® affinity media.
TEV protease is an engineered catalytic domain of the Tobacco Etch virus NIa protease. TEV protease is a highly specific cystein protease belonging to the C4 peptidase family.
Unit Definition
One unit of TEV protease cleaves >85% of 3 μg of control substrate in one hour at pH 8.0 at 30 °C.
Physical form
Supplied as a >=2 mg/ml in 25 mM Tris-HCl, pH 8.0, 50 mM NaCl, 1 mM TCEP, and 50% glycerol
aqueous glycerol solution
Preparation Note
Cleavage Protocol
Prepare fresh dialysis buffer. Dialysis buffer should be optimized for target protein solubility and contain no protease inhibitors. The dialysis buffer should also be compatible with downstream purification processes, e.g. minimal amount of EDTA or DTT if a HIS-Select® column will be used to remove the cleaved His-tag.
Example of suitable dialysis buffer: 25 mM Tris-HCl, pH 8.0, 150 - 500 mM NaCl, 14 mM β-mercaptoethanol
This TEV protease has the same activity in 150 mM NaCl or 500 mM NaCl and 400 mM imidazole.
Dilute the target protein sample to 1-2 mg/ml with dialysis buffer. This is optional in case the target protein aggregates in dialysis buffer. Save a small aliquot as a control for PAGE analysis. EDTA may be added to 0.5 mM final concentration if the target protein will be eluted from the HIS-Select® column and EDTA is compatible with the target protein.
Add TEV protease at a protease to target protein ratio of 1:100 (w/w) or 10,000 unit (1 mg) TEV protease to 100 mg of target protein. There is no need to calculate the molar ratio. TEV protease can be added directly to the target protein. There is no need to change buffer or dilute TEV protease. The optimal ratio should be determined empirically. A Protease-to-target protein ratio (w/w) of 1:50 to 1:200 should provide an affective range for most target proteins.
Dialyze against the dialysis buffer at 4 °C ~ 16 hrs. Dialysis is intended to remove imidazole or glutathione if HIS-Select® or glutathione affinity columns are used to remove the cleaved tag or TEV protease after cleavage.
Typically, 1 mg of TEV protease will cleave >90% of 100mg of a control protein at 4 °C in 16 hours.
Prepare fresh dialysis buffer. Dialysis buffer should be optimized for target protein solubility and contain no protease inhibitors. The dialysis buffer should also be compatible with downstream purification processes, e.g. minimal amount of EDTA or DTT if a HIS-Select® column will be used to remove the cleaved His-tag.
Example of suitable dialysis buffer: 25 mM Tris-HCl, pH 8.0, 150 - 500 mM NaCl, 14 mM β-mercaptoethanol
This TEV protease has the same activity in 150 mM NaCl or 500 mM NaCl and 400 mM imidazole.
Dilute the target protein sample to 1-2 mg/ml with dialysis buffer. This is optional in case the target protein aggregates in dialysis buffer. Save a small aliquot as a control for PAGE analysis. EDTA may be added to 0.5 mM final concentration if the target protein will be eluted from the HIS-Select® column and EDTA is compatible with the target protein.
Add TEV protease at a protease to target protein ratio of 1:100 (w/w) or 10,000 unit (1 mg) TEV protease to 100 mg of target protein. There is no need to calculate the molar ratio. TEV protease can be added directly to the target protein. There is no need to change buffer or dilute TEV protease. The optimal ratio should be determined empirically. A Protease-to-target protein ratio (w/w) of 1:50 to 1:200 should provide an affective range for most target proteins.
Dialyze against the dialysis buffer at 4 °C ~ 16 hrs. Dialysis is intended to remove imidazole or glutathione if HIS-Select® or glutathione affinity columns are used to remove the cleaved tag or TEV protease after cleavage.
Typically, 1 mg of TEV protease will cleave >90% of 100mg of a control protein at 4 °C in 16 hours.
Legal Information
HIS-Select is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service