455598
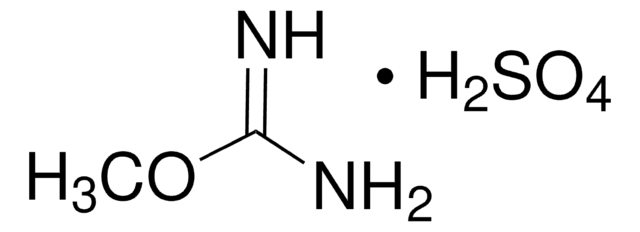
O-Methylisourea hemisulfate salt
99%
Synonym(s):
OMI®
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NC(OCH3)=NH · 1/2H2SO4
CAS Number:
Molecular Weight:
123.12
Beilstein:
3723107
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
163-167 °C (lit.)
solubility
water: soluble 100 mg/mL, clear, colorless
functional group
amine
SMILES string
COC(N)=N.COC(N)=N.OS(O)(=O)=O
InChI
1S/2C2H6N2O.H2O4S/c2*1-5-2(3)4;1-5(2,3)4/h2*1H3,(H3,3,4);(H2,1,2,3,4)
InChI key
QSCPQKVWSNUJLJ-UHFFFAOYSA-N
Gene Information
human ... OPRD1(4985), OPRK1(4986), OPRM1(4988)
rat ... Oprd1(24613), Oprm1(25601)
Looking for similar products? Visit Product Comparison Guide
Legal Information
OMI is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Optimization of guanidination procedures for MALDI mass mapping.
Beardsley RL and Reilly JP.
Analytical Chemistry, 74(8), 1884-1890 (2002)
John C Timmer et al.
The Biochemical journal, 407(1), 41-48 (2007-07-26)
Most known organisms encode proteases that are crucial for constitutive proteolytic events. In the present paper, we describe a method to define these events in proteomes from Escherichia coli to humans. The method takes advantage of specific N-terminal biotinylation of
V M Mahnir et al.
Toxicon : official journal of the International Society on Toxinology, 29(7), 819-826 (1991-01-01)
The effect of modification of amino groups on RTX-III induced lethality in mice has been studied. The toxicity was not affected by guanidination of one or two lysine residues with O-methylisourea, but guanidination of three or four lysine residues decreased
S I Shalabi et al.
The Journal of dairy research, 49(4), 607-617 (1982-11-01)
Several dicarbonyl compounds (glyoxal, substituted glyoxals, diacetyl and 1, 2-cyclohexanedione) had a marked stabilizing effect on the heat stability of milk, especially in the presence of urea. These reagents are believed to modify arginine more or less specifically suggesting an
M H Jouvin et al.
Journal of immunology (Baltimore, Md. : 1950), 133(6), 3250-3254 (1984-12-01)
Lysine epsilon-amino groups of human factor H were selectively converted to guanidino groups by treatment with 0.1 M O-methylisourea at pH 10.4. Guanidination resulted in a dose-dependent decrease in the capacity of the regulatory protein to accelerate decay dissociation of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service