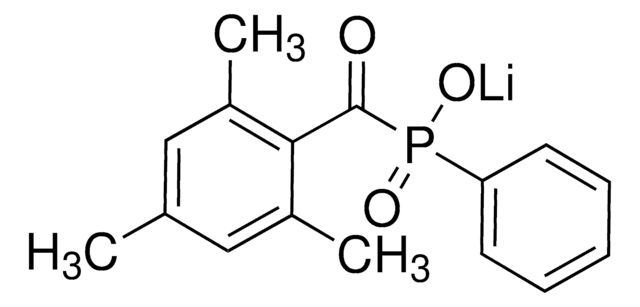
926035
TissueFab® bioink Bone UV/365 nm
Synonym(s):
3D Bioprinting, Bioink, GelMA, TissueFab
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352201
NACRES:
NA.23
Recommended Products
form
viscous liquid
Quality Level
impurities
<5 CFU/g Bioburden (Total Aerobic)
<5 CFU/g Bioburden (fungal)
<50 EU/mL Endotoxin
color
white
pH
6.5-7.5
viscosity
5-50 cP(37 °C)
application(s)
3D bioprinting
Related Categories
General description
TissueFab® bioink Bone Vis/365 nm, is designed for promoting osteogenic differentiation of stem cells. It is based on Gelatin methacryloyl (GelMA) - Hydroxyapatite (HAp) hydrogel system.
HAp is a highly crystalline form of calcium phosphate. HAp has a chemical similarity with the mineralized phase of bone which accounts for their excellent biocompatibility and osteoinductive and osteoconductive properties favorable for bone regeneration. HAp-containing hydrogels has been studied in literature to demonstrate their processability with different additive manufacturing approaches. Printing of cell laden structures with HAp containing bioink formulations have shown superior osteogenic properties.
Additional Information:
The protocol for this material can be found In the Documentation Section under ″More Documents″.
HAp is a highly crystalline form of calcium phosphate. HAp has a chemical similarity with the mineralized phase of bone which accounts for their excellent biocompatibility and osteoinductive and osteoconductive properties favorable for bone regeneration. HAp-containing hydrogels has been studied in literature to demonstrate their processability with different additive manufacturing approaches. Printing of cell laden structures with HAp containing bioink formulations have shown superior osteogenic properties.
Additional Information:
The protocol for this material can be found In the Documentation Section under ″More Documents″.
Application
TissueFab® bioink Bone UV/365 nm, low endotoxin is a ready-to-use bioink which is formulated for high cell viability, osteoinduction and printing fidelity and is designed for extrusion-based 3D bioprinting and subsequent crosslinking with exposure to 365 nm visible light. GelMA-Bone bioinks can be used with most extrusion-based bioprinters, are biodegradable, and are compatible with human mesenchymal stem cells (hMSCs) and osteogenic cell types. TissueFab® bioink Bone UV/365 nm, low endotoxin enables the precise fabrication of osteogenic 3D cell models and tissue constructs for research in 3D cell biology, tissue engineering, in vitro tissue models, and regenerative medicine.
Legal Information
TISSUEFAB is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service