F6889
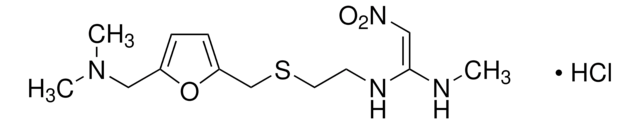
Famotidine
Synonym(s):
N′-(Aminosulfonyl)-3-([2-(diaminomethyleneamino)-4-thiazolyl]methylthio)propanamidine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H15N7O2S3
CAS Number:
Molecular Weight:
337.45
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
originator
Johnson & Johnson
SMILES string
N\C(N)=N\c1nc(CSCCC(=N)NS(N)(=O)=O)cs1
InChI
1S/C8H15N7O2S3/c9-6(15-20(12,16)17)1-2-18-3-5-4-19-8(13-5)14-7(10)11/h4H,1-3H2,(H2,9,15)(H2,12,16,17)(H4,10,11,13,14)
InChI key
XUFQPHANEAPEMJ-UHFFFAOYSA-N
Gene Information
human ... HRH2(3274)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Famotidine has been used to test its effect on the human ether-a-go-go-related gene (hERG) binding transfected in HEK293 membrane. It has also been used in polycaprolactone based drug delivery studies.
Biochem/physiol Actions
Famotidine is not effective on muscarinic and nicotinic receptors. It competitively inhibits the secretion of gastric acid and elicits mucosal injury protection.
H2 histamine receptor antagonist; anti-ulcer agent
Features and Benefits
This compound was developed by Johnson & Johnson. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Famotidine for the prevention of gastric and duodenal ulcers caused by nonsteroidal antiinflammatory drugs
Taha AS, et al.
The New England Journal of Medicine, 334(22), 1435-1439 (1996)
Makoto Anraku et al.
International journal of pharmaceutics, 487(1-2), 142-147 (2015-04-18)
An intermolecular complex formed from a 1:1 weight ratio of chitosan (CS, molecular weight 30 kDa) and sulfobutyl ether β-cyclodextrin (SBE-β-CyD, degree of substitution 7) was less soluble than either of the original components. The release of famotidine from tablets
Polycaprolactone thin-film drug delivery systems: empirical and predictive models for device design
Schlesinger E, et al.
Materials Science and Engineering, C, 57, 232-239 (2015)
Kenichi Yamamoto et al.
Journal of orthopaedic research : official publication of the Orthopaedic Research Society, 30(12), 1958-1962 (2012-05-18)
Heterotopic ossification or calcification follows any type of musculoskeletal trauma and is known to occur after arthroplasties of hip, knee, shoulder, or elbow; fractures; joint dislocations; or tendon ruptures. Histamine receptor H2 (Hrh2) has been shown to be effective for
H D Langtry et al.
Drugs, 38(4), 551-590 (1989-10-01)
Famotidine is a highly selective histamine H2-receptor antagonist. In healthy volunteers and patients with acid hypersecretory disease it is approximately 20 to 50 times more potent at inhibiting gastric acid secretion than cimetidine and 8 times more potent than ranitidine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service