328650
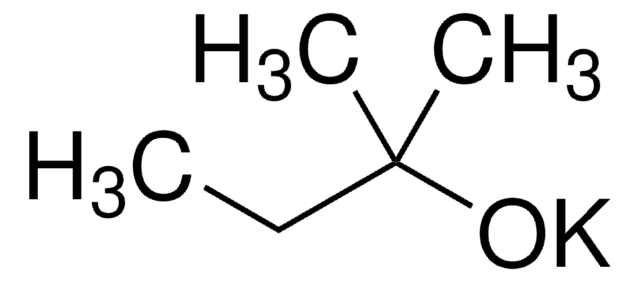
Potassium tert-butoxide solution
1.0 M in THF
동의어(들):
Potassium tert-butylate, Potassium-2-methylpropan-2-olate solution
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
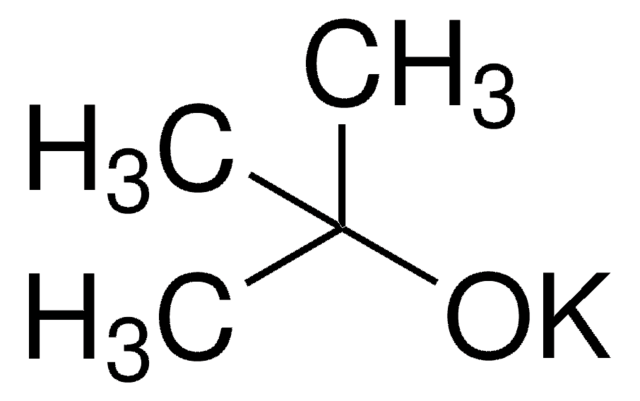
(CH3)3COK
CAS Number:
Molecular Weight:
112.21
Beilstein:
3556712
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.23
추천 제품
양식
liquid
Quality Level
반응 적합성
core: potassium
농도
1.0 M in THF
density
0.902 g/mL at 25 °C
SMILES string
[K+].CC(C)(C)[O-]
InChI
1S/C4H9O.K/c1-4(2,3)5;/h1-3H3;/q-1;+1
InChI key
LPNYRYFBWFDTMA-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Potassium tert-butoxide solution is a strong non nucleophilic base. It is capable of deprotonating carbon and other Bronsted acids. Reagent solutions in tert-BuOh may be prepared by reacting anhydrous alcohol with potassium in nitrogen atmosphere.
애플리케이션
Potassium tert-butoxide solution(t-BuOK) can be used:
- As a catalyst for the interesterification of rapeseed oil with methyl acetate.
- To promote Sommelet–Hauser rearrangements of N-benzylic amino acid-derived ammonium ylides under mild conditions.
- As a metal-organic precursor in the synthesis of KNbO3 ferroelectric films by the chemical vapor deposition method.
- To prepare 3-potassiooxamethylpyridine catalyst.
- As a strong alkoxide base reagent.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1A - STOT SE 3
표적 기관
Central nervous system, Respiratory system
보충제 위험성
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 1
Flash Point (°F)
-2.2 °F - closed cup
Flash Point (°C)
-19 °C - closed cup
이미 열람한 고객
One-step methodology for the synthesis of FA picolinyl esters from intact lipids.
Destaillats F and Angers P
Journal of the American Oil Chemists' Society, 79(3), 253-256 (2002)
Hajime Ito et al.
Chemical communications (Cambridge, England), 48(64), 8006-8008 (2012-07-10)
The regio- and diastereoselective silaboration of aromatic alkenes with a silylboron compound proceeds in the presence of a catalytic amount of potassium tert-butoxide, providing a complementary method to the corresponding transition metal-catalyzed reactions.
Aurélie Mallinger et al.
The Journal of organic chemistry, 74(3), 1124-1129 (2008-12-24)
3-Aryltetronic acids were prepared in one step by treatment of a mixture of methyl arylacetates and methyl hydroxyacetates with potassium tert-butoxide, via tandem transesterification/Dieckmann condensation. Several mushroom or lichen pigments, vulpinic acids, were synthesized from 3-(4-methoxyphenyl)tetronic acid in three steps
Scott E Denmark et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 12(19), 4954-4963 (2006-05-04)
This paper chronicles the conceptual development, proof of principle experiments, and recent advances in the palladium-catalyzed cross-coupling reactions of the conjugate bases of organosilanols. The discovery that led to the design and refinement of this process represents a classical illustration
Subhadip De et al.
Organic letters, 14(17), 4466-4469 (2012-08-16)
A transition-metal-free intramolecular dehydrohalide coupling via intramolecular homolytic aromatic substitution (HAS) with aryl radicals has been developed in the presence of potassium tert-butoxide and an organic molecule as the catalyst. The methodology has been applied to a concise synthesis of
문서
The properties of many devices are limited by the intrinsic properties of the materials that compose them.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.