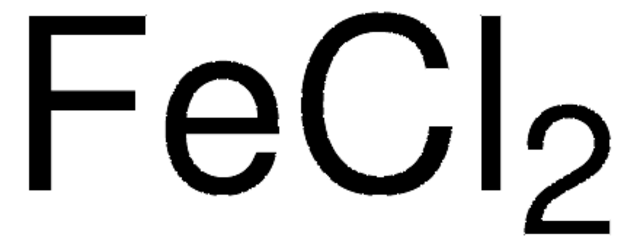추천 제품
Quality Level
분석
≥99.99% trace metals basis
양식
powder or crystals
환경친화적 대안 제품 특성
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
불순물
≤100 ppm (trace metals analysis)
mp
677 °C (lit.)
solubility
water: soluble
density
3.16 g/mL at 25 °C (lit.)
3.16 g/mL at 25 °C
음이온 미량물
chlorate, nitrate (as NO3-): ≤20 ppm
sulfate (SO42-): ≤20 ppm
≤50 ppm (oxygen)
양이온 미량물
Al: ≤10 ppm
B: ≤10 ppm
Ba: ≤10 ppm
Ca: ≤10 ppm
Co: ≤10 ppm
Cr: ≤10 ppm
Cu: ≤10 ppm
K: ≤10 ppm
Mg: ≤10 ppm
Mn: ≤10 ppm
Na: ≤10 ppm
Ni: ≤10 ppm
Si: ≤10 ppm
Ti: ≤10 ppm
Zn: ≤10 ppm
환경친화적 대안 카테고리
저장 온도
2-30°C
SMILES string
Cl[Fe]Cl
InChI
1S/2ClH.Fe/h2*1H;/q;;+2/p-2
InChI key
NMCUIPGRVMDVDB-UHFFFAOYSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
애플리케이션
특징 및 장점
- Anhydrous
- Low water content
- Low Oxygen content
-Perfect use for molten salt reactors
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.