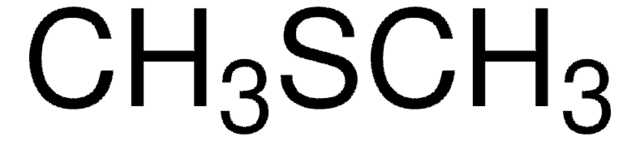모든 사진(3)
About This Item
Linear Formula:
(CH3)2S
CAS Number:
Molecular Weight:
62.13
Beilstein:
1696847
EC Number:
MDL number:
UNSPSC 코드:
12352112
PubChem Substance ID:
NACRES:
NA.21
bp:
38 °C (lit.)
vapor pressure:
26.24 psi ( 55 °C)
7.79 psi ( 20 °C)
7.79 psi ( 20 °C)
추천 제품
Grade
anhydrous
Quality Level
vapor density
2.1 (vs air)
vapor pressure
26.24 psi ( 55 °C)
7.79 psi ( 20 °C)
분석
≥99.0%
양식
liquid
autoignition temp.
402 °F
expl. lim.
19.7 %
불순물
<0.003% water
<0.005% water (100 mL pkg)
증발 잔류물
<0.0005%
refractive index
n20/D 1.435 (lit.)
bp
38 °C (lit.)
mp
−98 °C (lit.)
solubility
H2O: soluble 7.28 g/L at 20 °C
density
0.846 g/mL at 25 °C (lit.)
SMILES string
CSC
InChI
1S/C2H6S/c1-3-2/h1-2H3
InChI key
QMMFVYPAHWMCMS-UHFFFAOYSA-N
유전자 정보
rat ... Maoa(29253), Maob(25750)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Dimethyl sulfide is the major biogenic, volatile sulfur compound that is released from the ocean to the atmosphere. It plays a main role in the global sulfur cycle.
애플리케이션
Dimethyl sulfide undergoes thiolation reaction with hydrogen sulfide (H2S) in the presence of tungsten-zirconia (WO3/ZrO2) catalyst to form methanethiol.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
-32.8 °F - closed cup
Flash Point (°C)
-36 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves
이미 열람한 고객
Photooxidation of dimethyl sulfide and dimethyl disulfide. I: Mechanism development
Yin F, et al
Journal of Atmospheric Chemistry, 11(4), 309-364 (1990)
Ingrid Elisia et al.
PloS one, 11(3), e0152538-e0152538 (2016-04-01)
Dimethyl sulfoxide (DMSO) is currently used as an alternative treatment for various inflammatory conditions as well as for cancer. Despite its widespread use, there is a paucity of data regarding its safety and efficacy as well as its mechanism of
Thiolation of dimethyl sulfide to methanethiol over WO3/ZrO2 catalysts
Chen S, et al
J. Mol. Catal. A: Chem., 365, 60-65 (2012)
Thiolation of dimethyl sulfide to methanethiol over WO3/ZrO2 catalysts.
Chen S, et al.
J. Mol. Catal. A: Chem., 365, 60-65 (2012)
Dimethyl sulfide production during natural phytoplanktonic blooms.
Nguyen BC, et al.
Marine Chemistry, 24(2), 133-141 (1988)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.