646547
Tris(2-carboxyethyl)phosphine hydrochloride solution
0.5 M, pH 7.0(aqueous solution; pH was adjusted with ammonium hydroxide)
동의어(들):
TCEP
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C9H15O6P · HCl
CAS Number:
Molecular Weight:
286.65
MDL number:
UNSPSC 코드:
12352128
PubChem Substance ID:
NACRES:
NA.25
추천 제품
농도
0.5 M
refractive index
n20/D 1.367
pH
7.0(aqueous solution; pH was adjusted with ammonium hydroxide)
density
1.041 g/mL at 25 °C
SMILES string
Cl.OC(=O)CCP(CCC(O)=O)CCC(O)=O
InChI
1S/C9H15O6P.ClH/c10-7(11)1-4-16(5-2-8(12)13)6-3-9(14)15;/h1-6H2,(H,10,11)(H,12,13)(H,14,15);1H
InChI key
PBVAJRFEEOIAGW-UHFFFAOYSA-N
일반 설명
It belongs to the trialkylphosphine class.
Tris (2-carboxyethyl) phosphine (TCEP) is very effective in cleaving disulfide bonds in aqueous solution. It dissolves in water and is odorless, unlike other trialkylphosphines (tributylphosphine). It is also less toxic than 2-mercaptoethanol. These advantages make it better than the other reducing agents.
애플리케이션
Tris (2-carboxyethyl) phosphine (TCEP) can be used in several downstream applications including SDS-PAGE, mass spectrometry, labeling with cysteine specific tags, and modification of cysteine containing compounds. It prevents oxidation of protein samples, which makes it a useful buffer component as it helps to preserve enzymatic activity. It has been used in the reduction and measurement of glutathione.
Tris (2-carboxyethyl) phosphine (TCEP) has also been used:
- to cleave cysteine residues in a synthetic peptide
- in reduction buffer for RNA Sequential Probing of Targets (SPOTs) imaging
- for the reduction of oligonucleotides
- as reducing agent during mitochondrial isolation
생화학적/생리학적 작용
As a non-mercaptan reducing agent, it avoids the toxicity inherent in thiol-containing compounds. It is capable of disrupting the botulinum neurotoxin B heavy-chain/light-chain complex that is held together by a single disulfide bond, and that is responsible for endocytosis, and ultimately the toxicity, of the toxin. Since disulfide-coupled subunits are characteristic of many toxins (e.g., ricin, snake venom, and all BoNT serotypes), it may be useful as a rescue prophylactic in cases of toxin administration.
Tris(2-carboxyethyl)phosphine hydrochloride solution reduces the disulfide bonds and leaves other functional groups intact in proteins.
신호어
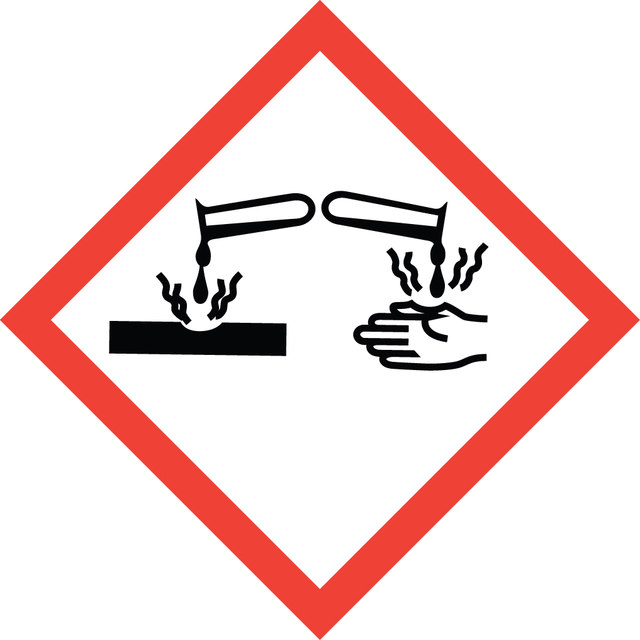
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
M C Gilbert Lee et al.
Journal of visualized experiments : JoVE, (87)(87), doi:10-doi:10 (2014-05-20)
Cell surface proteins, including extracellular matrix proteins, participate in all major cellular processes and functions, such as growth, differentiation, and proliferation. A comprehensive characterization of these proteins provides rich information for biomarker discovery, cell-type identification, and drug-target selection, as well
Defined DNA/nanoparticle conjugates
Ackerson CJ, et al.
Proceedings of the National Academy of Sciences of the USA, 102(38), 13383-13385 (2005)
Quantitative analysis of tris (2-carboxyethyl) phosphine by anion-exchange chromatography and evaporative light-scattering detection
Tan Z, et al.
Journal of Pharmaceutical and Biomedical Analysis, 59, 167-172 (2012)
Differential labeling of free and disulfide-bound thiol functions in proteins
Seiwert B, et al.
Journal of the American Society For Mass Spectrometry, 19(1), 1-7 (2008)
Highly multiplexed quantitative mass spectrometry analysis of ubiquitylomes
Rose CM, et al.
Cell Systems, 3(4), 395-403 (2016)
문서
In this study, we developed a rapid trypsin digest kit that, at elevated temperatures, yielded reliable, reproducible results in less than 2 hours on a wide variety of substrates for mass spectrometry.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.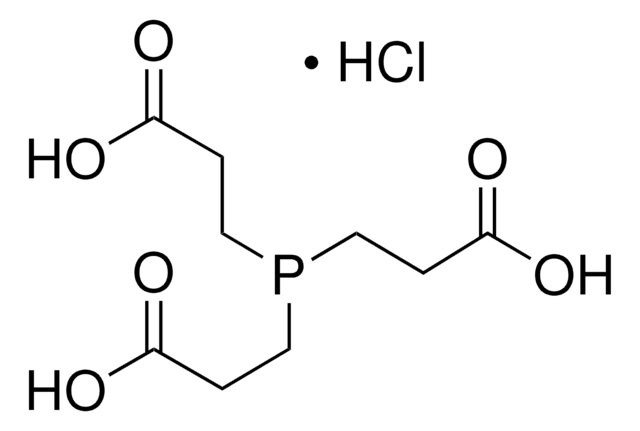




![Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)