추천 제품
Quality Level
분석
≥95%
양식
powder
분자량
491.18 g/mol
저장 온도
−20°C
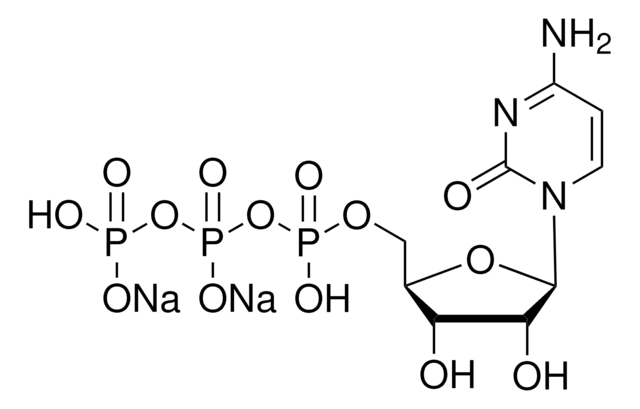
SMILES string
[Na].Nc1ncnc2n(cnc12)C3OC(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)CC3O
InChI
1S/C10H16N5O12P3.Na.H/c11-8-7-9(13-3-12-8)15(4-14-7)10-6(16)1-5(25-10)2-24-29(20,21)27-30(22,23)26-28(17,18)19;;/h3-6,10,16H,1-2H2,(H,20,21)(H,22,23)(H2,11,12,13)(H2,17,18,19);;
InChI key
FRGBONDYLMUOMH-UHFFFAOYSA-N
관련 카테고리
일반 설명
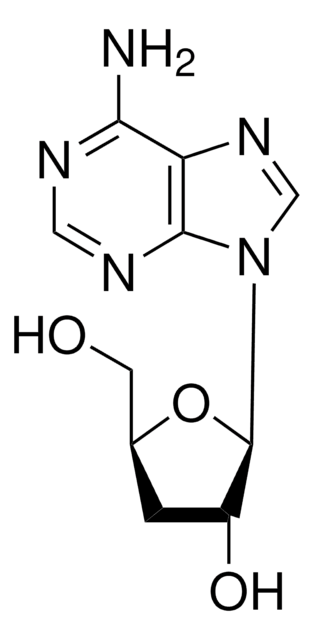
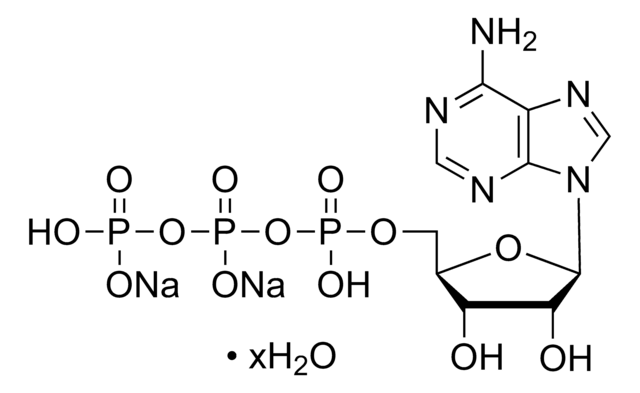
Cordycepin (3′-deoxyadenosine) is a cytotoxic adenosine analog and a bioactive agent extracted from Cordyceps militaris. Cordycepin 5′-triphosphate is a toxic derivative of cordycepin.
애플리케이션
Cordycepin 5′-triphosphate sodium salt has been used:
- as a transcriptional inhibitor in RNA decay assay
- to inhibit polyadenylation in HEK293T cells before harvesting for in- vitro interaction studies through co-immunoprecipitation (co-Ips)
- as a positive control in in vitro assays for polymerase inhibition
- as a precursor to produce cordycepin monophosphate
생화학적/생리학적 작용
Cordycepin 5′-triphosphate is incorporated into nucleic acid by poly(A) polymerase. Because it lacks a 3′-hydroxyl group chain production it can be used for 3′-end labeling of RNA.
Cordycepin exhibits various metabolic effects by activating AMP-activated protein kinase (AMPK) in humans. Cordycepin triphosphate inhibits picornavirus polymerase-specific RNA synthesis by blocking in vitro [3H]GMP incorporation. It also retards cell growth by inhibiting RNA synthesis.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
개인 보호 장비
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.