추천 제품
Grade
pharmaceutical primary standard
API family
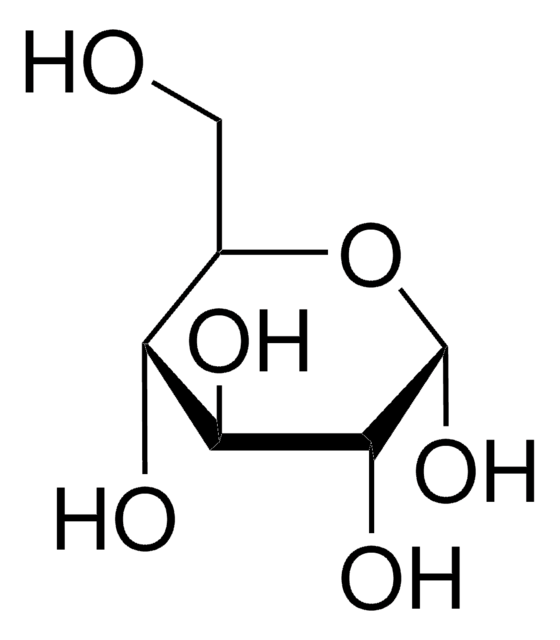
dextrose
양식
crystalline powder
제조업체/상표
USP
mp
150-152 °C (lit.)
응용 분야
pharmaceutical (small molecule)
형식
neat
SMILES string
OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3-,4+,5-,6+/m1/s1
InChI key
WQZGKKKJIJFFOK-DVKNGEFBSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Dextrose USP reference standard for specified quality tests and assay use.
Also used to prepare standard and system suitability solutions for the assay and identification tests according to the given below monographs of United States Pharmacopeia (USP):DextroseDextrose ExcipientLiquid GlucoseGalactoseDextrates.
The USP biologics carbohydrates category includes a variety of carbohydrate-based substances that are essential in the development and manufacturing of therapeutic products. These carbohydrates play crucial roles in biological processes and are often utilized as excipients, stabilizers, or active ingredients in pharmaceuticals. The USP provides comprehensive standards, reference materials, and analytical procedures to ensure the identity, quality, purity, and consistency of carbohydrate therapeutics throughout their lifecycle.
The United States Pharmacopeia (USP) provides quality standards for biologics to ensure their safety, efficacy, and quality throughout the manufacturing process. These standards assist manufacturers in adhering to regulatory requirements and help safeguard public health by reducing risks associated with biologics.
애플리케이션
Also used to prepare standard and system suitability solutions for the assay and identification tests according to the given below monographs of United States Pharmacopeia (USP):
- Dextrose
- Dextrose Excipient
- Liquid Glucose
- Galactose
- Dextrates
기타 정보
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.