추천 제품
Grade
pharmaceutical primary standard
API family
oligosaccharide sst mixture
양식
solid
제조업체/상표
USP
응용 분야
pharmaceutical (small molecule)
형식
mixture
일반 설명
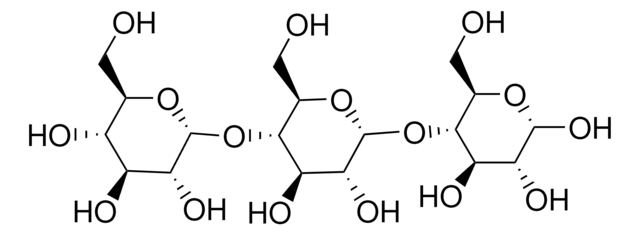
Oligosaccharide System Suitability Mixture A is composed of structurally distinct carbohydrate fragments used in glycan analysis. It is employed to assess chromatographic resolution, system performance, and method suitability for oligosaccharide profiling.
The USP biologics carbohydrates category includes a variety of carbohydrate-based substances that are essential in the development and manufacturing of therapeutic products. These carbohydrates play crucial roles in biological processes and are often utilized as excipients, stabilizers, or active ingredients in pharmaceuticals. The USP provides comprehensive standards, reference materials, and analytical procedures to ensure the identity, quality, purity, and consistency of carbohydrate therapeutics throughout their lifecycle.
The United States Pharmacopeia (USP) provides quality standards for biologics to ensure their safety, efficacy, and quality throughout the manufacturing process. These standards assist manufacturers in adhering to regulatory requirements and help safeguard public health by reducing risks associated with biologics.
애플리케이션
Also used to prepare AB and APTS-labeled standard solutions for liquid chromatographic and capillary electrophoresis separation labeling.
Additionally, used to separate simple biantennary N-linked oligosaccharide chains with no or low levels of sialylated structures.
분석 메모
기타 정보
관련 제품
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.