1615138
USP
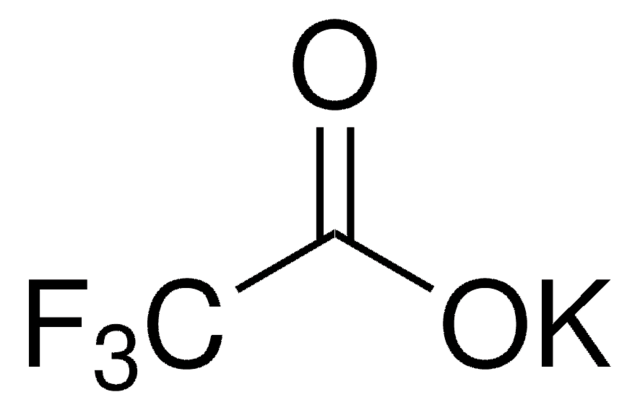
Sodium trifluoroacetate
United States Pharmacopeia (USP) Reference Standard
동의어(들):
Sodium trifluoroacetate, Trifluoroacetic acid sodium salt
About This Item
추천 제품
Grade
pharmaceutical primary standard
API family
sodium trifluoroacetate
양식
powder
제조업체/상표
USP
mp
205-207 °C (dec.) (lit.)
density
1.49 g/mL (lit.)
응용 분야
pharmaceutical (small molecule)
형식
neat
SMILES string
[Na+].[O-]C(=O)C(F)(F)F
InChI
1S/C2HF3O2.Na/c3-2(4,5)1(6)7;/h(H,6,7);/q;+1/p-1
InChI key
UYCAUPASBSROMS-UHFFFAOYSA-M
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
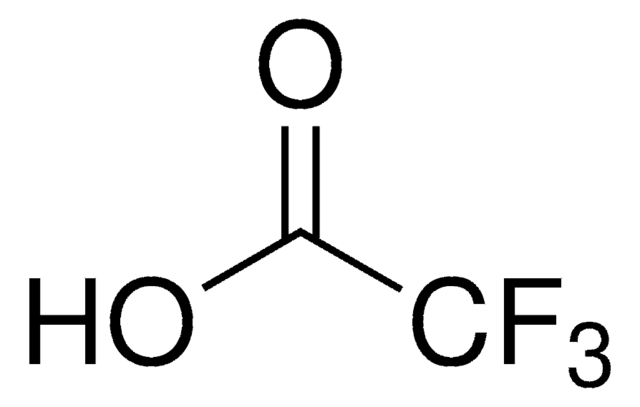
Sodium trifluoroacetate (TFA) is a commonly encountered residual reagent or counter-ion in peptide synthesis and purification. It is often present due to the use of trifluoroacetic acid in cleavage and purification steps. The USP standard supports the identification and quantification of TFA in peptide active pharmaceutical ingredients (APIs) and formulations.
The USP biologics peptides category encompasses a diverse range of therapeutic peptides that are essential in managing various medical conditions. These peptides, typically consisting of amino acid sequences of 40 residues or less, are critical for the development of high-quality medicines. The USP provides comprehensive standards, reference materials, and analytical procedures to ensure the identity, quality, purity, and consistency of peptide therapeutics throughout their lifecycle.
The United States Pharmacopeia (USP) provides quality standards for biologics to ensure their safety, efficacy, and quality throughout the manufacturing process. These standards assist manufacturers in adhering to regulatory requirements and help safeguard public health by reducing risks associated with biologics.
애플리케이션
Also used to prepare trifluoroacetic acid (TFA) Stock solution to determine the amount of trifluoroacetic acid (TFA) in peptides according to the given below general chapter of United States Pharmacopeia (USP):
- 〈503.1〉 Trifluoroacetic acid (TFA) in peptides
기타 정보
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Aquatic Chronic 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.