추천 제품
Grade
pharmaceutical primary standard
API family
vancomycin
양식
solid
제조업체/상표
USP
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
−20°C
일반 설명
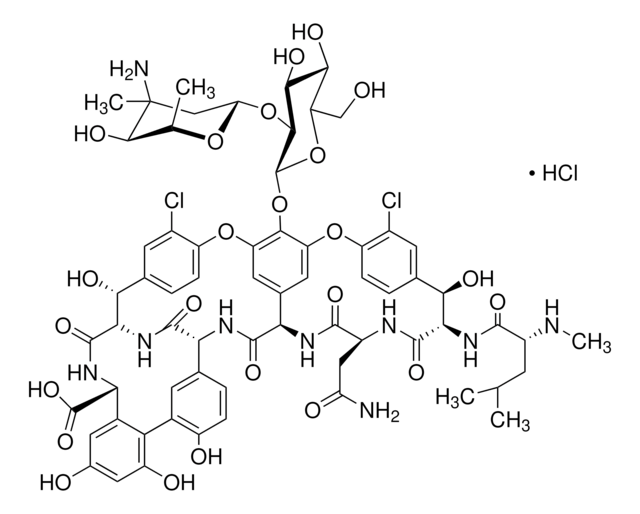
Vancomycin hydrochloride is a glycopeptide antibiotic effective against Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA). It inhibits cell wall biosynthesis by binding to D-Ala-D-Ala termini of cell wall precursors. The USP standard provides a validated benchmark for microbiological assays and chromatographic identification techniques.
The USP biologics antibiotics category includes a wide range of antimicrobial agents that are essential in treating bacterial infections. These antibiotics are derived from various natural sources or synthesized to combat specific pathogens effectively. The USP provides comprehensive standards, reference materials, and analytical procedures to ensure the identity, quality, purity, and consistency of antibiotic therapeutics throughout their lifecycle.
The United States Pharmacopeia (USP) provides quality standards for biologics to ensure their safety, efficacy, and quality throughout the manufacturing process. These standards assist manufacturers in adhering to regulatory requirements and help safeguard public health by reducing risks associated with biologics.
애플리케이션
- Vancomycin Hydrochloride Capsules
- Vancomycin Hydrochloride for Injection
- Vancomycin Hydrochloride for Oral Solution
- Vancomycin Injection
- Vancomycin
포장
기타 정보
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.