125237
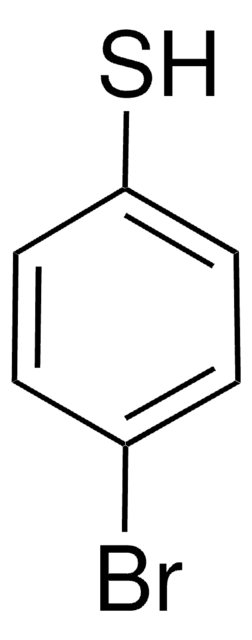
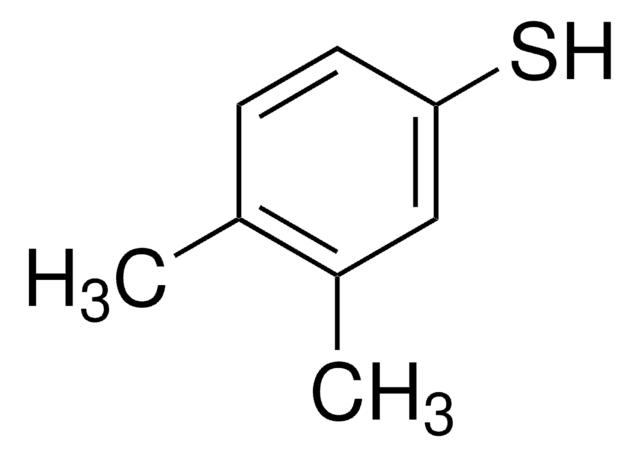
4-Chlorothiophenol
97%
Synonym(s):
4-Chlorobenzenethiol, 4-Chlorophenyl mercaptan
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
ClC6H4SH
CAS Number:
Molecular Weight:
144.62
Beilstein:
605971
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
bp
205-207 °C (lit.)
mp
49-51 °C (lit.)
solubility
methanol: soluble
functional group
chloro
SMILES string
Sc1ccc(Cl)cc1
InChI
1S/C6H5ClS/c7-5-1-3-6(8)4-2-5/h1-4,8H
InChI key
VZXOZSQDJJNBRC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Chlorothiophenol undergoes oxidative coupling catalyzed by iron(III)-tetra phenyl prophyrin to form disulfides.
Application
4-Chlorothiophenol was used to modify poly(vinyl chloride) and to synthesize poly(vinyl chloride) with halogen groups.
Reactant involved in:
- Oxidation to disulfides using cobalt-salen catalysts and air oxidizing agents
- Thiourea-catalyzed sulfa-Michael addition to acryloyloxazolidinones
- Pummerer-type cyclization
- C-H Activation and C-S cross-coupling with heterocycles
- Allylic sulfenylation with allylic carbonates
- Lewis acid-catalyzed electrophilic ring-opening reactions of 2-aryl-3,4-dihydropyrans
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service