132756
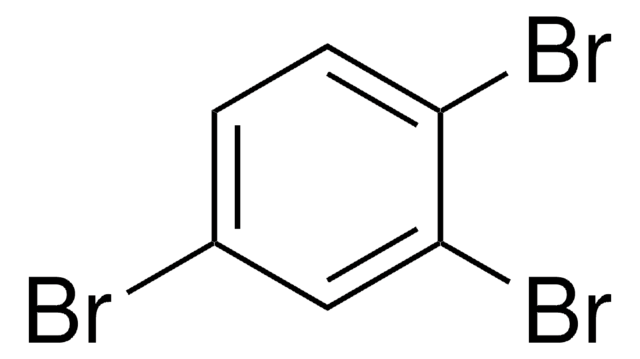
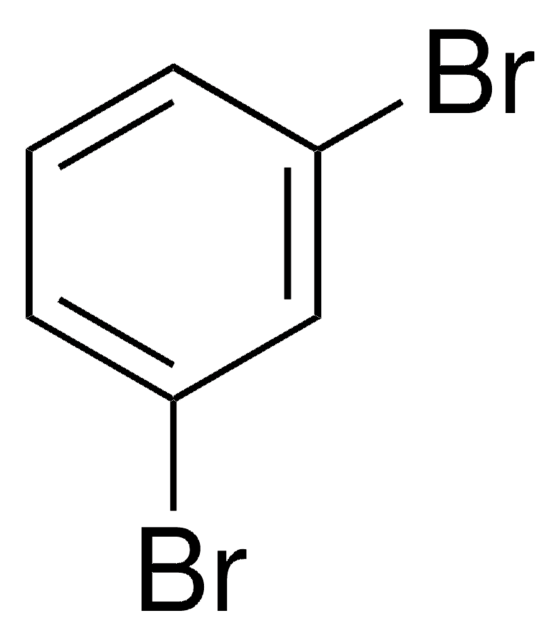
1,2,4-Tribromobenzene
95%
Sign Into View Organizational & Contract Pricing
About This Item
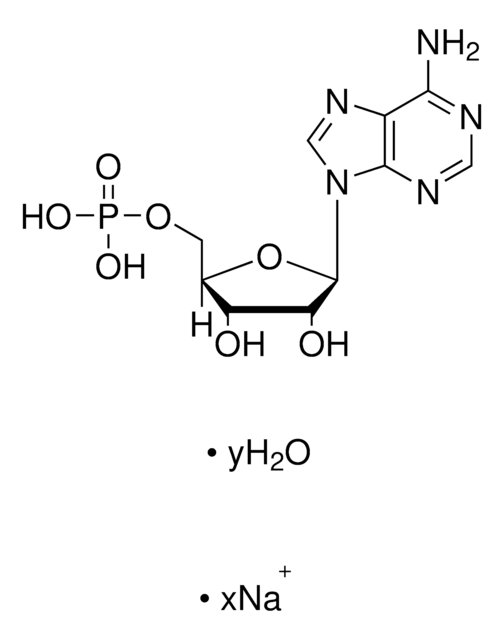
Empirical Formula (Hill Notation):
C6H3Br3
CAS Number:
Molecular Weight:
314.80
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Pricing and availability is not currently available.
Recommended Products
Quality Level
Assay
95%
form
solid
mp
41-43 °C (lit.)
functional group
bromo
SMILES string
Brc1ccc(Br)c(Br)c1
InChI
1S/C6H3Br3/c7-4-1-2-5(8)6(9)3-4/h1-3H
InChI key
FWAJPSIPOULHHH-UHFFFAOYSA-N
Related Categories
1 of 4
This Item | 194395 | 523429 | 438782 |
|---|---|---|---|
| assay 95% | assay 97% | assay 97% | assay 97% |
| Quality Level 100 | Quality Level 100 | Quality Level 100 | Quality Level 100 |
| mp 41-43 °C (lit.) | mp −7 °C (lit.) | mp 218-222 °C (lit.) | mp - |
| form solid | form liquid | form - | form liquid |
| functional group bromo | functional group bromo | functional group bromo, ketone | functional group bromo, fluoro |
General description
1,2,4-tribromobenzene on photochemical dehalogenation in open-air solutions of acetonitrile yields 1,4-dibromobenzene, 1,3-dibromobenzene and 1,2-dibromobenzene[1].
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Fluorination of alcohols and diols with a novel fluorous deoxy-fluorination reagent.
Furuya T, et al.
Journal of Fluorine Chemistry, 130(2), 348-353 (2009)
Halogen Exchange Reaction of Aliphatic Fluorine Compounds with Organic Halides as Halogen Source.
Mizukami Y, et al.
Organic Letters, 17(24), 5942-5945 (2015)
Regioselective Hydroxylation of C12?C15 Fatty Acids with Fluorinated Substituents by Cytochrome P450 BM3.
Chiang, Chih-Hsiang, et al.
Chemistry?A European Journal, 19(41), 13680-13691 (2013)
Vapor Pressures and Boiling Points of the 1-Fluoroalkanes, 1-Chloroalkanes, 1-Bromoalkanes, and 1-Iodoalkanes, C1to C20.
Li JCM and Rossini RD.
Journal of Chemical and Engineering Data, 6(2), 268-270 (1961)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service