233625
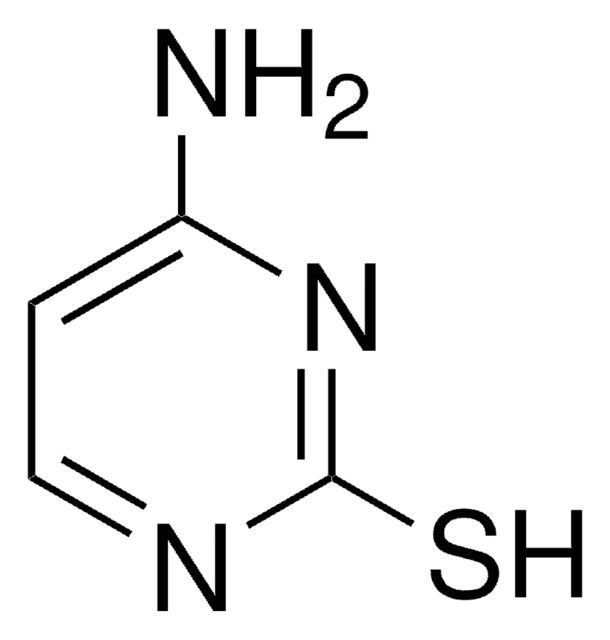
2-Thiocytosine
97%
Synonym(s):
4-Amino-2-mercaptopyrimidine, 4-Amino-2-thiopyrimidine
Sign Into View Organizational & Contract Pricing
Select a Size
Change View
50 MG
₩207,162
About This Item
Empirical Formula (Hill Notation):
C4H5N3S
CAS Number:
Molecular Weight:
127.17
Beilstein:
112435
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
285-290 °C (dec.) (lit.)
SMILES string
NC1=NC(=S)NC=C1
InChI
1S/C4H5N3S/c5-3-1-2-6-4(8)7-3/h1-2H,(H3,5,6,7,8)
InChI key
DCPSTSVLRXOYGS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
1 of 4
This Item | 765880 | 217727 | 402028 |
|---|---|---|---|
| assay 97% | assay 97% | assay 97% | assay 97% |
| Quality Level 100 | Quality Level 100 | Quality Level 100 | Quality Level 100 |
| mp 285-290 °C (dec.) (lit.) | mp 58-62 °C | mp - | mp 249 °C (dec.) (lit.) |
General description
Application
2-Thiocytosine has been used to investigate the reactions involved in hole transfer (HT) in X-irradiated doped cytosine monohydrate[2].
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
L A Poirier et al.
Regulatory toxicology and pharmacology : RTP, 30(3), 217-222 (2000-01-06)
Recently, changes have been proposed in the criteria historically used in the evaluation of the applicability to humans of some of the results obtained from the rodent carcinogenicity bioassay data. These questions center on the suitability of the rodent model
Anna Gulkowska et al.
Chemosphere, 107, 366-372 (2014-01-28)
Soil incubation experiments using (14)C-labelled sulfamethazine were carried out to assess the factors governing its nonextractable residue (NER) formation via nucleophilic addition reactions. Circumstantial evidence on possible mechanisms of NER formation was derived from a selective manipulation of soil samples.
Astrid Bienert-Zeit et al.
American journal of veterinary research, 76(4), 318-327 (2015-03-31)
To monitor concentrations of sulfadimidine in the paranasal sinus mucosa (PSM) of unsedated horses following IV administration of trimethoprim-sulfadimidine via in vivo microdialysis. 10 healthy adult horses. Concentric microdialysis probes were implanted into the subepithelial layers of the frontal sinus
Jie Hou et al.
Environmental science and pollution research international, 22(6), 4545-4554 (2014-10-17)
A feasible and rapid analysis for the simultaneous determination of sulfonamides (SAs), tetracyclines (TCs), fluoroquinolones (FQs), macrolides (MACs) and nitrofurans (NFs) in livestock manure and soils was established by solid-phase extraction (SPE)-ultra-performance liquid chromatography (UPLC)-tandem mass spectrometry (MS/MS). A total
Zhe Meng et al.
Food chemistry, 174, 597-605 (2014-12-23)
A novel UPLC-MS/MS method for quantification and confirmation of eight fluoroquinolones, five sulphonamides (SAs) and four acetyled metabolites in milk is developed. Their main fragmentation pathways were presented. The limits of quantification were in the range of 0.01-0.29 μg kg(-1)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service