All Photos(1)
About This Item
Empirical Formula (Hill Notation):
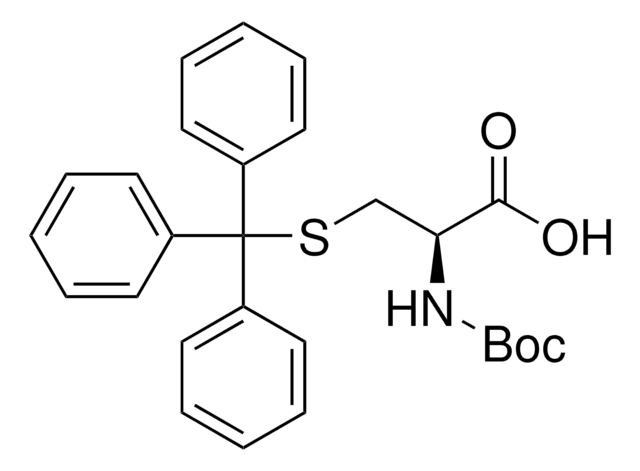
C20H20N2O6S2
CAS Number:
Molecular Weight:
448.51
Beilstein:
2068210
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
optical activity
[α]20/D −215±8°, c = 1% in ethanol
reaction suitability
reaction type: solution phase peptide synthesis
mp
195-200 °C (dec.)
application(s)
peptide synthesis
SMILES string
OC(=O)[C@H](CSSC[C@H](NC(=O)c1ccccc1)C(O)=O)NC(=O)c2ccccc2
InChI
1S/C20H20N2O6S2/c23-17(13-7-3-1-4-8-13)21-15(19(25)26)11-29-30-12-16(20(27)28)22-18(24)14-9-5-2-6-10-14/h1-10,15-16H,11-12H2,(H,21,23)(H,22,24)(H,25,26)(H,27,28)/t15-,16-/m0/s1
InChI key
GUTXMPQWQSOAIY-HOTGVXAUSA-N
Related Categories
Application
Reactant involved in:
- Synthesis of integrin antagonists
- Dissipative self-assembly of molecular gelator using chemical fuel
- Synthesis of crosslinkable hydrogel for proliferation of encapsulated human derm fibroblast
- Analysis for use as metallo-β-lactamase inhibitors
- Amplification of bifunctional disulfide ligands for calmodulin
Other Notes
Chiral modifier of LiBH4 for enantioselective reductions of aromatic, heteroaromatic and unsaturated ketones to alcohols
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service