689785
(S)-3,3′-Bis(2,4,6-triisopropylphenyl)-1,1′-binaphthyl-2,2′-diyl hydrogenphosphate
≥97.0% (qNMR)
Synonym(s):
(11bS)-4-Hydroxy-2,6-bis[2,4,6-tris(1-methylethyl)phenyl]dinaphtho[2,1-d:1′,2′-f]-1,3,2-dioxaphosphepin 4-oxide, (S)-3,3′-Bis(2,4,6-triisopropylphenyl)-1,1′-bi-2-naphthol cyclic monophosphate, (S)-TRIP
Select a Size
About This Item
Recommended Products
Quality Level
Assay
≥97.0% (qNMR)
form
lumps
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
functional group
phosphate
greener alternative category
, Aligned
SMILES string
CC(C)c1cc(C(C)C)c(c(c1)C(C)C)-c2cc3ccccc3c-4c2OP(O)(=O)Oc5c(cc6ccccc6c-45)-c7c(cc(cc7C(C)C)C(C)C)C(C)C
InChI
1S/C50H57O4P/c1-27(2)35-23-39(29(5)6)45(40(24-35)30(7)8)43-21-33-17-13-15-19-37(33)47-48-38-20-16-14-18-34(38)22-44(50(48)54-55(51,52)53-49(43)47)46-41(31(9)10)25-36(28(3)4)26-42(46)32(11)12/h13-32H,1-12H3,(H,51,52)
InChI key
AGQAQYPGJDBIQR-UHFFFAOYSA-N
General description
Application
Metal-Brønsted Acid Cooperative Catalysis for Asymmetric Reductive Amination
Packaging
1 of 4
This Item | SAB4701030 | SAB4701012 | SAB4500087 |
|---|---|---|---|
| antibody form affinity isolated antibody | antibody form purified immunoglobulin | antibody form purified immunoglobulin | antibody form affinity isolated antibody |
| Quality Level 100 | Quality Level - | Quality Level - | Quality Level 100 |
| conjugate unconjugated | conjugate unconjugated | conjugate unconjugated | conjugate unconjugated |
| biological source rabbit | biological source mouse | biological source mouse | biological source rabbit |
| storage temp. −20°C | storage temp. 2-8°C | storage temp. 2-8°C | storage temp. −20°C |
| mol wt antigen 51 kDa | mol wt - | mol wt - | mol wt antigen 50 kDa |
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
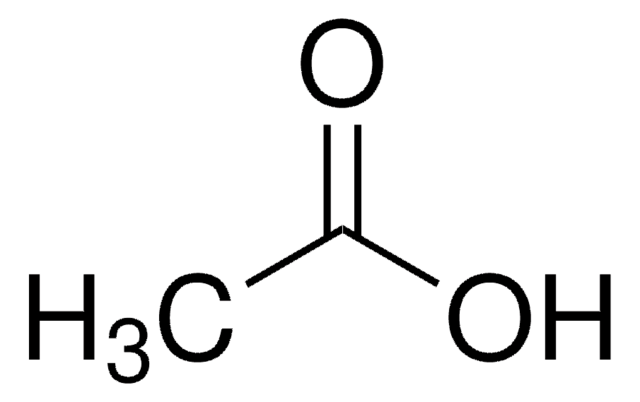
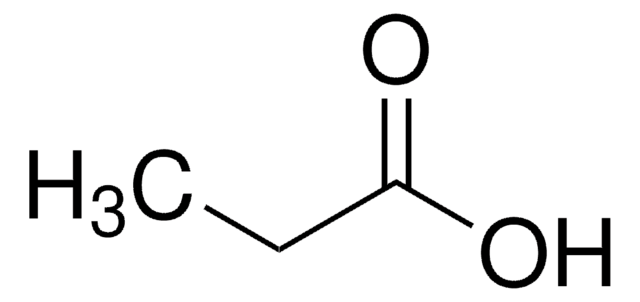
Separation of Propionic acid; Acetic acid; Heptanoic acid; Isobutyric acid; Valeric acid; Isocaproic acid; Butyric acid; Isovaleric acid
Separation of Methyl oleate; Caprylic acid; Heptanoic acid; Methyl decanoate; Methyl dodecanoate; Myristic acid; Methyl palmitate; Methyl palmitoleate; Methyl stearate; Methyl linoleate; Methyl linolenate; Acetic acid; Arachidic acid; Behenic acid; Propionic acid; Isobutyric acid; Valeric acid; Isovaleric acid; Isocaproic acid; Butyric acid
Butyl methyl ether; Acetic acid; 2-Butanone; Ethyl acetate; Tetrahydrofuran; 1-Butanol; Isopropyl acetate; Heptane; Propyl acetate; 3-Methylbutanol; 4-Methyl-2-pentanone; Isobutyl acetate; Butyl acetate; Dimethyl sulfoxide; Anisole; Cumene
Protocols
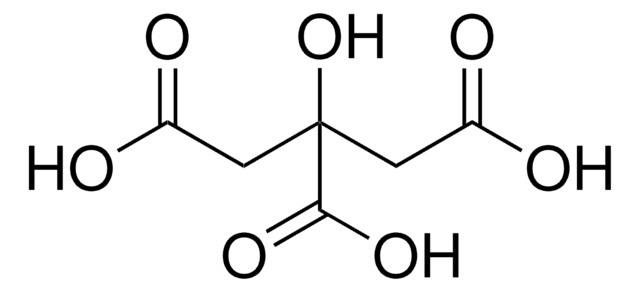
Separation of Pyruvic acid, United States Pharmacopeia (USP) Reference Standard; Tartaric acid, United States Pharmacopeia (USP) Reference Standard; Citric acid, United States Pharmacopeia (USP) Reference Standard; Malic acid, United States Pharmacopeia (USP) Reference Standard; L-Pyroglutamic acid, ≥99.0% (T); Lactic acid, United States Pharmacopeia (USP) Reference Standard; Acetic acid, ≥99.99% trace metals basis; Succinic acid, United States Pharmacopeia (USP) Reference Standard
In this study, SPME was used for the analysis of free fatty acids in Parmesan cheese using a 65 μm Carbowax/divinylbenzene (DVB) SPME fiber. Headspace extraction of the cheese sample was conducted at 65 °C for 15 minutes and analyzed by GC with FID detection. SPME is ideal for analyzing the volatiles associated with solid food samples. The phase chemistry of the Nukol GC column provides excellent peak shape of acidic compounds.
Separation of Acetone; Acetic acid; Propionic acid; Ethyl butyrate; Ethanol; Isoamyl acetate; Isobutyric acid; 3-Methyl-2-butanol; Methyl acetate; 1-Propanol; Acetal, ≥98%, FG; 2-Methyl-1-pentanol; Butyl acetate; Ethyl propionate; 3-Pentanol; 2-Pentanol, 98%; Ethyl isobutyrate; Isobutyl acetate; Acetaldehyde; Furfural; Butyric acid; Methanol; Ethyl acetate