C1M15W
MPAGE®® Comb
1.0 mm, 15 wells, for use with MPAGE® Gel Caster
About This Item
Recommended Products
Product Name
mPAGE® Combs 1.0 mm 15 Wells, 5pk, for use with mPAGE® Gel Caster
packaging
pkg of 5 packs
manufacturer/tradename
Millipore
technique(s)
SDS-PAGE: suitable
electrophoresis: suitable
W × H
10.1 cm × 8.3 cm
gel size
8.3 cm × 10.1 cm
gel thickness
1.0 mm
well size
15 wells
well volume
26 μL
compatibility
for use with mPAGE® Gel Caster components:
1 of 4
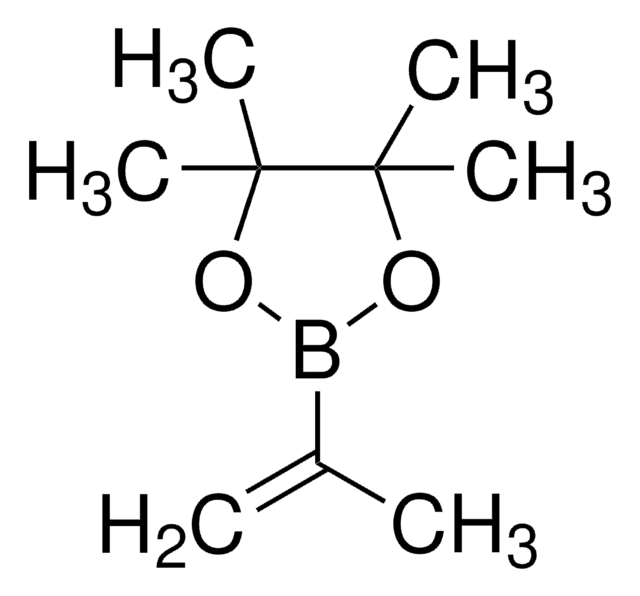
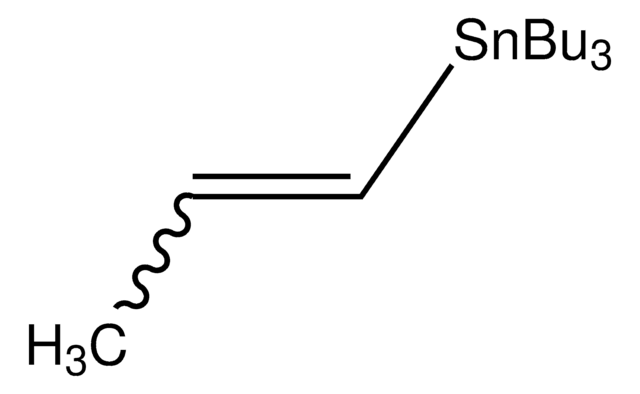
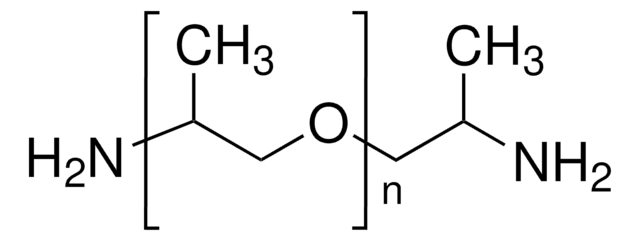
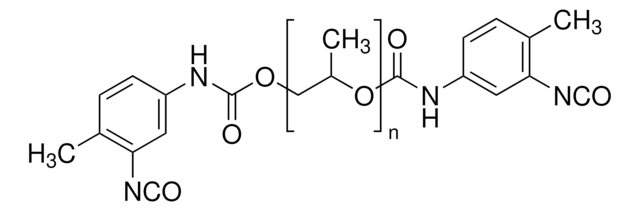
This Item | 408352 | 433497 | 438111 |
|---|---|---|---|
| refractive index n20/D 1.451 | refractive index n20/D 1.449 | refractive index n20/D 1.4742 | refractive index n20/D 1.448 |
| form viscous liquid | form liquid | form viscous liquid | form viscous liquid |
| density 0.996 g/mL at 25 °C | density 1.01 g/mL at 25 °C (lit.) | density 1.05 g/mL at 25 °C | density 0.989 g/mL at 25 °C |
| mol wt average Mn ~2,000 | mol wt average Mn ~375 | mol wt average Mn ~2,300 (narrow MW distribution), degree of polymerization ~34 | mol wt average Mn ~1,000 |
| Quality Level 100 | Quality Level - | Quality Level 100 | Quality Level 100 |
General description
Legal Information
related product
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service